Electrified bioreactors: the next power-up for biometallurgical wastewater treatment
- PMID: 34927376
- PMCID: PMC8913880
- DOI: 10.1111/1751-7915.13992
Electrified bioreactors: the next power-up for biometallurgical wastewater treatment
Abstract
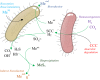
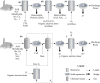
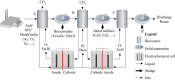
Over the past decades, biological treatment of metallurgical wastewaters has become commonplace. Passive systems require intensive land use due to their slow treatment rates, do not recover embedded resources and are poorly controllable. Active systems however require the addition of chemicals, increasing operational costs and possibly negatively affecting safety and the environment. Electrification of biological systems can reduce the use of chemicals, operational costs, surface footprint and environmental impact when compared to passive and active technologies whilst increasing the recovery of resources and the extraction of products. Electrification of low rate applications has resulted in the development of bioelectrochemical systems (BES), but electrification of high rate systems has been lagging behind due to the limited mass transfer, electron transfer and biomass density in BES. We postulate that for high rate applications, the electrification of bioreactors, for example, through the use of electrolyzers, may herald a new generation of electrified biological systems (EBS). In this review, we evaluate the latest trends in the field of biometallurgical and microbial-electrochemical wastewater treatment and discuss the advantages and challenges of these existing treatment technologies. We advocate for future research to focus on the development of electrified bioreactors, exploring the boundaries and limitations of these systems, and their validity upon treating industrial wastewaters.
© 2021 The Authors. Microbial Biotechnology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Abin, C.A. , and Hollibaugh, J.T. (2014) Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism. Environ Sci Technol 48: 681–688. - PubMed
-
- Adams, D.J. , Peoples, M. & Opara, A. (2012) A new electro‐biochemical reactor (EBR) for treatment of wastewaters. Water in Mineral Processing, pp. 2–12.
-
- Agostino, V. , and Rosenbaum, M.A. (2018) Sulfate‐reducing electroautotrophs and their applications in bioelectrochemical systems. Front Energy Res 6: 55.
-
- Anaya‐Garzon, J. , Mosquera‐Romero, S. , Leon‐Fernandez, L.F. , Garcia‐Timermans, C. , Van Dorpe, J. & Hoorens, A. Chromium nano‐mining with electrified bioreactors (to be published).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

