Mechanisms and physiological implications of cooperative gating of clustered ion channels
- PMID: 34927454
- PMCID: PMC8934683
- DOI: 10.1152/physrev.00022.2021
Mechanisms and physiological implications of cooperative gating of clustered ion channels
Abstract
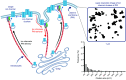
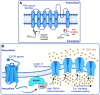
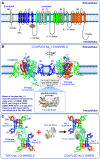
Ion channels play a central role in the regulation of nearly every cellular process. Dating back to the classic 1952 Hodgkin-Huxley model of the generation of the action potential, ion channels have always been thought of as independent agents. A myriad of recent experimental findings exploiting advances in electrophysiology, structural biology, and imaging techniques, however, have posed a serious challenge to this long-held axiom, as several classes of ion channels appear to open and close in a coordinated, cooperative manner. Ion channel cooperativity ranges from variable-sized oligomeric cooperative gating in voltage-gated, dihydropyridine-sensitive CaV1.2 and CaV1.3 channels to obligatory dimeric assembly and gating of voltage-gated NaV1.5 channels. Potassium channels, transient receptor potential channels, hyperpolarization cyclic nucleotide-activated channels, ryanodine receptors (RyRs), and inositol trisphosphate receptors (IP3Rs) have also been shown to gate cooperatively. The implications of cooperative gating of these ion channels range from fine-tuning excitation-contraction coupling in muscle cells to regulating cardiac function and vascular tone, to modulation of action potential and conduction velocity in neurons and cardiac cells, and to control of pacemaking activity in the heart. In this review, we discuss the mechanisms leading to cooperative gating of ion channels, their physiological consequences, and how alterations in cooperative gating of ion channels may induce a range of clinically significant pathologies.
Keywords: calcium signaling; channel clustering; cooperative gating; excitability; stochastic self-assembly.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
-
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc., 2001, p. 814.
-
- Sato D, Hernández-Hernández G, Matsumoto C, Tajada S, Moreno CM, Dixon RE, O’Dwyer S, Navedo MF, Trimmer JS, Clancy CE, Binder MD, Santana LF. A stochastic model of ion channel cluster formation in the plasma membrane. J Gen Physiol 151: 1116–1134, 2019. doi:10.1085/jgp.201912327. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS110953/NS/NINDS NIH HHS/United States
- R01 HL128537/HL/NHLBI NIH HHS/United States
- OT2 OD026580/OD/NIH HHS/United States
- R01 HL152681/HL/NHLBI NIH HHS/United States
- R01 HL161872/HL/NHLBI NIH HHS/United States
- R01 NS114210/NS/NINDS NIH HHS/United States
- R01 HL144071/HL/NHLBI NIH HHS/United States
- R01 HL085686/HL/NHLBI NIH HHS/United States
- R01 HL098200/HL/NHLBI NIH HHS/United States
- R01 AG063796/AG/NIA NIH HHS/United States
- R01 HL149127/HL/NHLBI NIH HHS/United States
- R01 HL121059/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

