Concussion susceptibility is mediated by spreading depolarization-induced neurovascular dysfunction
- PMID: 34927674
- PMCID: PMC9246711
- DOI: 10.1093/brain/awab450
Concussion susceptibility is mediated by spreading depolarization-induced neurovascular dysfunction
Abstract
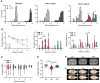
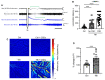
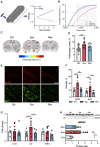
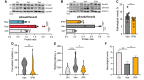
The mechanisms underlying the complications of mild traumatic brain injury, including post-concussion syndrome, post-impact catastrophic death, and delayed neurodegeneration remain poorly understood. This limited pathophysiological understanding has hindered the development of diagnostic and prognostic biomarkers and has prevented the advancement of treatments for the sequelae of mild traumatic brain injury. We aimed to characterize the early electrophysiological and neurovascular alterations following repetitive mild traumatic brain injury and sought to identify new targets for the diagnosis and treatment of individuals at risk of severe post-impact complications. We combined behavioural, electrophysiological, molecular, and neuroimaging techniques in a rodent model of repetitive mild traumatic brain injury. In humans, we used dynamic contrast-enhanced MRI to quantify blood-brain barrier dysfunction after exposure to sport-related concussive mild traumatic brain injury. Rats could clearly be classified based on their susceptibility to neurological complications, including life-threatening outcomes, following repetitive injury. Susceptible animals showed greater neurological complications and had higher levels of blood-brain barrier dysfunction, transforming growth factor β (TGFβ) signalling, and neuroinflammation compared to resilient animals. Cortical spreading depolarizations were the most common electrophysiological events immediately following mild traumatic brain injury and were associated with longer recovery from impact. Triggering cortical spreading depolarizations in mild traumatic brain injured rats (but not in controls) induced blood-brain barrier dysfunction. Treatment with a selective TGFβ receptor inhibitor prevented blood-brain barrier opening and reduced injury complications. Consistent with the rodent model, blood-brain barrier dysfunction was found in a subset of human athletes following concussive mild traumatic brain injury. We provide evidence that cortical spreading depolarization, blood-brain barrier dysfunction, and pro-inflammatory TGFβ signalling are associated with severe, potentially life-threatening outcomes following repetitive mild traumatic brain injury. Diagnostic-coupled targeting of TGFβ signalling may be a novel strategy in treating mild traumatic brain injury.
Keywords: biomarker; blood–brain barrier; concussion; dynamic contrast-enhanced MRI; repetitive mild traumatic brain injury.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Mortimer JA, Van Duijn CM, Chandra V, et al. . Head trauma as a risk factor for Alzheimer’s disease: A collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20:S28–S35. - PubMed
-
- Ben-Shlomo Y. The epidemiology of Parkinson’s disease. Baillieres Clin Neurol. 1997;6:55–68. - PubMed
-
- Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006;60:65–72. - PubMed

