Hypertension in pregnancy and adverse outcomes among low-risk nulliparous women expectantly managed at or after 39 weeks: a secondary analysis of a randomised controlled trial
- PMID: 34927787
- PMCID: PMC9207156
- DOI: 10.1111/1471-0528.17059
Hypertension in pregnancy and adverse outcomes among low-risk nulliparous women expectantly managed at or after 39 weeks: a secondary analysis of a randomised controlled trial
Abstract
Objective: To evaluate whether hypertensive disorders of pregnancy (HDP) among low-risk nulliparous women expectantly managed at or after 39 weeks of gestation are associated with adverse outcomes.
Design: Secondary analysis of a randomised trial.
Setting: Multicentre, USA.
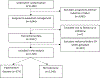
Population: Individuals in the expectantly managed group who delivered on or after 39 weeks.
Methods: Multivariable analysis to estimate adjusted relative risks (aRR) for binomial outcomes, adjusted odds ratios (aOR) for multinomial outcomes and 95% CI.
Main outcome measures: Composite adverse maternal outcome including placental abruption, pulmonary oedema, postpartum haemorrhage, postpartum infection, venous thromboembolism or intensive care unit admission. Secondary outcomes included a composite of perinatal death or severe neonatal complications, mode of delivery, small and large for gestational age and neonatal intermediate or intensive unit length of stay.
Results: Of the 3044 women randomised to expectant management in the original trial, 2718 (89.3%) were eligible for this analysis, of whom 373 (13.7%) developed HDP. Compared with participants who remained normotensive, those who developed HDP were more likely to experience the maternal composite (12% versus 6%, aRR 1.84, 95% CI 1.33-2.54) and caesarean delivery (29% versus 23%, aOR 1.32, 95% CI 1.01-1.71). Differences between the two groups were not significantly different for the adverse perinatal composite (7% versus 5%, aRR 1.38, 95% CI 0.92-2.07) or for other secondary outcomes.
Conclusion: Almost 14% of low-risk nulliparous individuals expectantly managed at 39 weeks developed HDP, and were more likely to experience adverse maternal outcomes compared with those who did not develop HDP.
Tweetable abstract: Almost 14% of low-risk nulliparous individuals expectantly managed at 39 weeks developed hypertensive disorders of pregnancy, and were more likely to experience adverse maternal outcomes compared with those who did not develop hypertensive disorders.
Keywords: adverse maternal outcome; adverse neonatal outcome; caesarean delivery; expectant management; hypertensive disorders of pregnancy; induction of labour; pre-eclampsia.
© 2021 John Wiley & Sons Ltd.
Conflict of interest statement
Disclosures of interest:
None declared. Completed disclosure of interest forms are available to view online as supporting information.
Figures
Comment in
-
Hypertensive disorders of pregnancy: term and conditions.BJOG. 2022 Jul;129(8):1404-1405. doi: 10.1111/1471-0528.17080. Epub 2022 Jan 10. BJOG. 2022. PMID: 34954892 No abstract available.
References
-
- UT Health- University of Texas Medical School at Houston--Children’s Memorial Hermann Hospital, Houston, TX: – Ortiz F, Chauhan S, Garcia L (Harris Health System, Lyndon B. Johnson Hospital; ),
-
- Sibai B Northwestern University, Chicago, IL: – Mallett G, Grobman W, Peaceman A, Plunkett B(NorthShore University; ),
-
- Paycheck K (NorthShore University), Dinsmoor M (NorthShore University; )
-
- University of Alabama at Birmingham, Birmingham, AL: – Harris S, Sheppard J, Biggio J, Harper L, Longo S (Ochsner; ), C. Servay (Ochsner)
-
- University of Utah Health Sciences Center, Salt Lake City, UT: – Hill K, Varner M, Sowles A, Coleman K (Utah Valley Hospital; ), D. Atkinson (Utah Valley Hospital), J. Stratford (McKay-Dee Hospital), S. Dellermann (McKay-Dee Hospital), C. Meadows (McKay-Dee Hospital), S. Esplin (Intermountain Med Ctr), C. Martin (Intermountain Med Ctr), K. Peterson (Intermountain Med Ctr), S. Stradling (LDS Hospital)
Publication types
MeSH terms
Grants and funding
- HD40545/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD40560/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- HD40500/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- HD68282/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD040545/HD/NICHD NIH HHS/United States
- HD34208/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD040500/HD/NICHD NIH HHS/United States
- HD87192/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD068268/HD/NICHD NIH HHS/United States
- HD40544/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UG1 HD034116/HD/NICHD NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- HD27869/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UG1 HD053097/HD/NICHD NIH HHS/United States
- HD34116/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- HD68258/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD040485/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD53097/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD034116/HD/NICHD NIH HHS/United States
- UG1 HD068258/HD/NICHD NIH HHS/United States
- HD87230/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- HD40512/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- HD68268/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD027869/HD/NICHD NIH HHS/United States
- HD40485/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD027915/HD/NICHD NIH HHS/United States
- U10 HD068282/HD/NICHD NIH HHS/United States
- UL1TR001873/TR/NCATS NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- UG1 HD068268/HD/NICHD NIH HHS/United States
- U10 HD36801/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- U10 HD040560/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- U10 HD068258/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD068282/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- P2C HD050924/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- HD27915/Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources
Medical