Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex
- PMID: 34928427
- PMCID: PMC9047512
- DOI: 10.1007/s00401-021-02393-1
Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex
Abstract
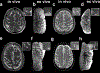
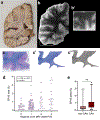
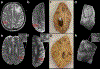
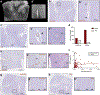
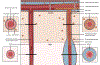
Perivascular spaces (PVS) are compartments surrounding cerebral blood vessels that become visible on MRI when enlarged. Enlarged PVS (EPVS) are commonly seen in patients with cerebral small vessel disease (CSVD) and have been suggested to reflect dysfunctional perivascular clearance of soluble waste products from the brain. In this study, we investigated histopathological correlates of EPVS and how they relate to vascular amyloid-β (Aβ) in cerebral amyloid angiopathy (CAA), a form of CSVD that commonly co-exists with Alzheimer's disease (AD) pathology. We used ex vivo MRI, semi-automatic segmentation and validated deep-learning-based models to quantify EPVS and associated histopathological abnormalities. Severity of MRI-visible PVS during life was significantly associated with severity of MRI-visible PVS on ex vivo MRI in formalin fixed intact hemispheres and corresponded with PVS enlargement on histopathology in the same areas. EPVS were located mainly around the white matter portion of perforating cortical arterioles and their burden was associated with CAA severity in the overlying cortex. Furthermore, we observed markedly reduced smooth muscle cells and increased vascular Aβ accumulation, extending into the WM, in individually affected vessels with an EPVS. Overall, these findings are consistent with the notion that EPVS reflect impaired outward flow along arterioles and have implications for our understanding of perivascular clearance mechanisms, which play an important role in the pathophysiology of CAA and AD.
Keywords: Cerebral amyloid angiopathy; Cerebral small vessel disease; Clearance; Enlarged perivascular spaces; Ex vivo MRI.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

References
-
- Atzeni A, Jansen M, Ourselin S, Iglesias JE (2018) A probabilistic model combining deep learning and multi-atlas segmentation for semi-automated labelling of histology. Lect Notes Comput Sci 11071:219–227. doi: 10.1007/978-3-030-00934-2_25 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

