RELAY, Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients with Untreated, Epidermal Growth Factor Receptor Mutation-Positive, Metastatic Non-Small-Cell Lung Cancer: Safety Profile and Manageability
- PMID: 34928484
- PMCID: PMC8763844
- DOI: 10.1007/s40264-021-01127-2
RELAY, Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients with Untreated, Epidermal Growth Factor Receptor Mutation-Positive, Metastatic Non-Small-Cell Lung Cancer: Safety Profile and Manageability
Abstract
Introduction: RELAY was a global, double-blind, placebo-controlled phase III study that demonstrated superior progression-free survival (PFS) for ramucirumab plus erlotinib (RAM + ERL) versus placebo plus erlotinib (PBO + ERL) in the first-line treatment of patients with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 (L858R) mutation-positive, metastatic non-small-cell lung cancer (NSCLC).
Objective: This article provides an in-depth analysis of the safety profile of RAM + ERL versus PBO + ERL observed in RELAY.
Methods: Eligible patients met these criteria: stage IV NSCLC; EGFR exon 19 deletion or exon 21 substitution (L858R) mutation; Eastern Cooperative Oncology Group performance status 0 or 1; and no central nervous system metastases. Patients were randomized (1:1) to receive erlotinib 150 mg/day orally plus either ramucirumab 10 mg/kg intravenously or matching placebo once every 2 weeks, until disease progression or unacceptable toxicity. The primary endpoint was PFS. Safety was evaluated based on reported treatment-emergent adverse events (AEs) and clinical laboratory assessments.
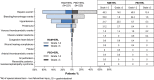
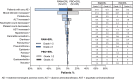
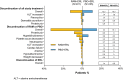
Results: The safety population comprised 446 patients (221 in RAM+ERL arm; 225 in PBO + ERL arm) who received at least one dose of study drug between January 2016 and February 2018. The overall incidence of grade ≥ 3 AEs was higher with RAM + ERL than with PBO + ERL, primarily driven by grade 3 hypertension. Grade ≥ 3 dermatitis acneiform and diarrhea were also reported more frequently in the RAM + ERL arm. The increased incidence of AEs with RAM + ERL was easily detected through routine monitoring and managed through dose adjustments and appropriate supportive care.
Conclusion: This in-depth safety analysis from RELAY supports that RAM + ERL, irrespective of the increased incidence of AEs, does not affect a patient's ability to benefit from treatment.
Clinical trial registration number: NCT02411448.
© 2021. The Author(s).
Conflict of interest statement
EN has received research grants from Bristol Myers Squibb, Merck Serono, Roche, and Pfizer; advisory board fees from Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Amgen, Pfizer, Eli Lilly and Company, Takeda, Sanofi, Bayer, AstraZeneca, and Merck Serono; and lecturing/presentation/writing payments from Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Amgen, Pfizer, Eli Lilly and Company, Takeda, Bayer, AstraZeneca, and Boehringer Ingelheim. HH has received lecture fees, honoraria, or other fees from Eli Lilly and Company, AstraZeneca, Merck Sharp & Dohme, Ono, and Bristol Myers Squibb and research grants from Merck Sharp & Dohme, Chugai, Ono, Bristol Myers Squibb, AstraZeneca, Daiichi Sankyo, Novartis, and Genomic Health. JYS has served as an advisory board member for AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Chugai Pharma, Ono Pharmaceutical, Takeda, CStone Pharmaceuticals, Janssen, and Bristol Myers Squibb; has received speaking honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Chugai Pharma, Ono Pharmaceutical, and Bristol Myers Squibb; and has received a research grant from Roche. KN has received grants and personal fees from AstraZeneca K.K., Astellas Pharma, Merck Sharp & Dohme K.K., Ono Pharmaceutical, Nippon Boehringer Ingelheim, Novartis Pharma K.K., Pfizer Japan, Bristol Myers Squibb, Eli Lilly Japan K.K., Chugai Pharmaceutical, Daiichi Sankyo, and Merck Serono; personal fees from Medicus Shuppan, Publishers Co., Ltd, Care Net, Reno, Kyorin, Roche Diagnostics K.K., Bayer Yakuhin, Medical Mobile Communications Co., 3H Clinical Trial, Nanzando, Yodosha, Nikkei Business Publications, Thermo Fisher Scientific K.K., Yomiuri Telecasting Corporation, Nippon Kayaku, and Nichi-Iko Pharmaceutical; grants and personal fees from Takeda Pharmaceutical, Taiho Pharmaceutical, SymBio Pharmaceuticals, and AbbVie; and grants from inVentiv Health Japan, Icon Japan K.K., Gritstone Oncology, Parexel International, Kissei Pharmaceutical, EPS Corporation, Syneos Health, Pfizer R&D Japan G.K., A2 Healthcare, Quintiles Inc/IQVIA Services Japan K.K., EP-CRSU, Linical, Eisai, CMIC Shift Zero K.K., Kyowa Hakko Kirin, Bayer Yakuhin, EPS International, and Otsuka Pharmaceutical. MR has received personal fees from Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung. EBG has received grants from AstraZeneca, Dynavax, Eli Lilly and Company, Genentech, Iovance, Mirati and Neon; grants and personal fees from Bristol Myers Squibb, EMD Serono, Merck, and Novartis; and personal fees from ABL, Boehringer Ingelheim, Dracen, Eisai, GSK, Sanofi, Shionogi, and Xilio. Y-FW has no conflicts of interest that are directly relevant to the content of this article. JK has served as an advisory board member for, without receiving any personal fees from, Roche Pharma, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Takeda, Astra Zeneca, Amgen, and Eli Lilly and Company. BFM, EB, OL, and CVG are employees of and own stock in Eli Lilly and Company. SN has received speaker bureau/advisor personal fees from Amgen, AstraZeneca, Boehringer, Beigene, Merck Sharp & Dohme, Roche, Sanofi, Novartis, Takeda, and Pfizer.
Figures






References
-
- Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210. doi: 10.1093/annonc/mdy554. - DOI - PubMed
-
- Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, Phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. - DOI - PubMed
-
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, Phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

