Parahydrogen-Induced Polarization in Hydrogenation Reactions Mediated by a Metal-Free Catalyst
- PMID: 34928532
- PMCID: PMC9303582
- DOI: 10.1002/chem.202103501
Parahydrogen-Induced Polarization in Hydrogenation Reactions Mediated by a Metal-Free Catalyst
Abstract
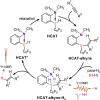
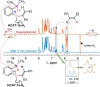
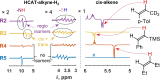
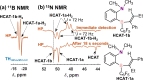
We report nuclear spin hyperpolarization of various alkenes achieved in alkyne hydrogenations with parahydrogen over a metal-free hydroborane catalyst (HCAT). Being an intramolecular frustrated Lewis pair aminoborane, HCAT utilizes a non-pairwise mechanism of H2 transfer to alkynes that normally prevents parahydrogen-induced polarization (PHIP) from being observed. Nevertheless, the specific spin dynamics in catalytic intermediates leads to the hyperpolarization of predominantly one hydrogen in alkene. PHIP enabled the detection of important HCAT-alkyne-H2 intermediates through substantial 1 H, 11 B and 15 N signal enhancement and allowed advanced characterization of the catalytic process.
Keywords: Lewis acids; Lewis bases; NMR hyperpolarization; organocatalysis; parahydrogen.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Levitt M. H., Spin Dynamics: Basics of Nuclear Magnetic Resonance , 2nd ed., Wiley, Chichester, 2008.
-
- None
-
- Hövener J. B., Pravdivtsev A. N., Kidd B., Bowers C. R., Glöggler S., Kovtunov K. V., Plaumann M., Katz-Brull R., Buckenmaier K., Jerschow A., Reineri F., Theis T., Shchepin R. V., Wagner S., Bhattacharya P., Zacharias N. M., Chekmenev E. Y., Angew. Chem. Int. Ed. 2018, 57, 11140–11162; - PMC - PubMed
- Angew. Chem. 2018, 130, 11310–11333;
-
- Barskiy D. A., Coffey A. M., Nikolaou P., Mikhaylov D. M., Goodson B. M., Branca R. T., Lu G. J., Shapiro M. G., Telkki V.-V., Zhivonitko V. V., Koptyug I. V., Salnikov O. G., Kovtunov K. V., Bukhtiyarov V. I., Rosen M. S., Barlow M. J., Safavi S., Hall I. P., Schröder L., Chekmenev E. Y., Chem. Eur. J. 2017, 23, 725–751. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

