COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study
- PMID: 34928700
- PMCID: PMC8697485
- DOI: 10.7326/M21-3256
COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study
Abstract
Background: Little is known about real-world COVID-19 vaccine effectiveness (VE) in racially and ethnically diverse, elderly populations with high comorbidity burden.
Objective: To determine the effectiveness of messenger RNA COVID-19 vaccines.
Design: Target trial emulation study comparing newly vaccinated persons with matched unvaccinated controls.
Setting: U.S. Department of Veterans Affairs health care system.
Participants: Among persons receiving care in the Veterans Affairs health care system (n = 5 766 638), those who received at least 1 dose of the Moderna or Pfizer-BioNTech COVID-19 vaccine from 11 December 2020 to 25 March 2021 (n = 2 099 871) were matched to unvaccinated controls in a 1:1 ratio according to demographic, clinical, and geographic characteristics.
Intervention: Follow-up for SARS-CoV-2 infection or SARS-CoV-2-related death, defined as death within 30 days of infection, began after the vaccination date or an identical index date for the matched unvaccinated controls and continued until up to 30 June 2021.
Measurements: Vaccine effectiveness against SARS-CoV-2 infection or SARS-CoV-2-related death.
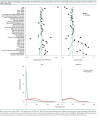
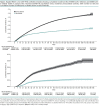
Results: Vaccinated and unvaccinated groups were well matched; both were predominantly male (92.9% vs. 93.4%), had advanced age (mean, 68.7 years in both groups), had diverse racial and ethnic distribution (for example, Black: 17.3% vs. 17.0%, Hispanic: 6.5% vs. 6.1%), and had substantial comorbidity burden. Vaccine effectiveness 7 or more days after the second vaccine dose was 69% (95% CI, 67% to 70%) against SARS-CoV-2 infection and 86% (CI, 82% to 89%) against SARS-CoV-2-related death and was similar when follow-up was extended to 31 March versus 30 June. Vaccine effectiveness against infection decreased with increasing age and comorbidity burden.
Limitation: Predominantly male population and lack of data on SARS-CoV-2 variants.
Conclusion: In an elderly, diverse, high-comorbidity population, COVID-19 VE against infection was substantially lower than previously reported, but VE against death was high. Complementary infection mitigation efforts remain important for pandemic control, even with vaccination.
Primary funding source: U.S. Department of Veterans Affairs.
Conflict of interest statement
Figures

References
-
- Hall VJ , Foulkes S , Saei A , et al; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725-1735. [PMID: ] doi:10.1016/S0140-6736(21)00790-X - DOI - PMC - PubMed
-
- Thompson MG , Burgess JL , Naleway AL , et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495-500. [PMID: ] doi:10.15585/mmwr.mm7013e3 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
