Health-Related Quality of Life in Metastatic, Hormone-Sensitive Prostate Cancer: ENZAMET (ANZUP 1304), an International, Randomized Phase III Trial Led by ANZUP
- PMID: 34928708
- PMCID: PMC8906451
- DOI: 10.1200/JCO.21.00941
Health-Related Quality of Life in Metastatic, Hormone-Sensitive Prostate Cancer: ENZAMET (ANZUP 1304), an International, Randomized Phase III Trial Led by ANZUP
Abstract
Purpose: We previously reported that enzalutamide improved overall survival when added to standard of care in metastatic, hormone-sensitive prostate cancer. Here, we report its effects on aspects of health-related quality of life (HRQL).
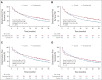
Methods: HRQL was assessed with the European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire and QLM-PR25 at weeks 0, 4, 12, and then every 12 weeks until progression. Scores from week 4 to 156 were analyzed with repeated measures modeling to calculate group means and differences. Deterioration-free survival was from random assignment until the earliest of death, clinical progression, discontinuation of study treatment, or a worsening of 10 points or more from baseline in fatigue, physical function, cognitive function, or overall health and quality of life (OHQL). HRQL scores range from 0 (lowest possible) to 100 (highest possible).
Results: HRQL was assessed in 1,042 of 1,125 participants (93%). Differences in means favored control over enzalutamide for fatigue (5.2, 95% CI, 3.6 to 6.9; P < .001), cognitive function (4.0, 95% CI, 2.5 to 5.5; P < .001), and physical function (2.6, 95% CI, 1.3 to 3.9; P < .001), but not OHQL (1.2, 95% CI, -0.2 to 2.7; P = .1). Deterioration-free survival rates at 3 years, and log-rank P values comparing the whole distributions, favored enzalutamide over control for OHQL (31% v 17%; P < .0001), cognitive function (31% v 20%; P = .001), and physical function (31% v 22%; P < .001), but not fatigue (24% v 18%; P = .16). The effects of enzalutamide on HRQL were independent of baseline characteristics.
Conclusion: Enzalutamide was associated with worsening of self-reported fatigue, cognitive function, and physical function, but not OHQL. Enzalutamide was associated with improved deterioration-free survival for OHQL, physical function, and cognitive function because delays in disease progression outweighed early deteriorations in these aspects of HRQL.
Trial registration: ClinicalTrials.gov NCT02446405.
Conflict of interest statement
Figures


Comment in
-
Elevating the Patient Voice in Metastatic Hormone-Sensitive Prostate Cancer Clinical Trials.J Clin Oncol. 2022 Mar 10;40(8):807-810. doi: 10.1200/JCO.21.02504. Epub 2022 Jan 6. J Clin Oncol. 2022. PMID: 34990219 No abstract available.
-
Reply to L. Marandino et al.J Clin Oncol. 2022 Jul 1;40(19):2175-2176. doi: 10.1200/JCO.22.00337. Epub 2022 Apr 21. J Clin Oncol. 2022. PMID: 35446593 No abstract available.
-
Impact of Novel Hormonal Therapy on Cognitive Function: Essential to Measure, Difficult to Present.J Clin Oncol. 2022 Jul 1;40(19):2174-2175. doi: 10.1200/JCO.22.00092. Epub 2022 Apr 21. J Clin Oncol. 2022. PMID: 35446611 No abstract available.
References
-
- Scher HI, Fizazi K, Saad F, et al. ; Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 - PubMed
-
- Davis ID, Martin AJ, Stockler MR, et al. : ENZAMET trial investigators and the Australian and New Zealand urogenital and prostate cancer trials group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 381:121-131, 2019 - PubMed
-
- Piccirillo JF, Tierney RM, Costas I, et al. : Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291:2441-2447, 2004 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

