Can one-step nucleic acid amplification assay predict four or more positive axillary lymph node involvement in breast cancer patients: a single-centre retrospective study
- PMID: 34928727
- PMCID: PMC10334894
- DOI: 10.1308/rcsann.2021.0154
Can one-step nucleic acid amplification assay predict four or more positive axillary lymph node involvement in breast cancer patients: a single-centre retrospective study
Abstract
Background: One-step nucleic acid amplification (OSNA) assay is a proven, accurate, intraoperative method for the detection of lymph node (LN) metastases. The aim of this study was to assess if the total tumour load (TTL) as calculated by OSNA could be used to predict N2 stage disease, ie ≥4 LN containing metastases, in invasive breast cancer patients.
Methods: Between 2011 and 2019 at St Richard's Hospital, Chichester, all macro-metastasis-positive OSNA cases for invasive breast cancer were retrospectively reviewed. The association between clinicopathological variables and ≥4 LNs containing metastases was analysed using regression analysis.
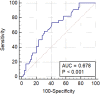
Results: In total, 134 patients with positive sentinel lymph node (SLN) on OSNA undergoing axillary node clearance were analysed, 53% of whom had no further positive LN, 25% had ≥4 lymph nodes positive. TTL was calculated as the aggregate of cytokeratin-19 mRNA copy count of all SLN tissue analysed via OSNA. TTL ≥1.1×105copies/μl and lymphovascular invasion (LVI) were both significant predictors of N2 stage disease on both univariate (TTL p=0.04, LVI p=0.005) and multivariate (TTL p=0.008, LVI p=0.039) regression analysis.
Conclusion: Our findings show that SLN TTL via intraoperative OSNA assay can predict four or more positive axillary LN involvement in invasive breast cancer. This is important in that it may be used intraoperatively by surgeons to decide on whether to proceed with a full axillary node clearance in order to stage the axilla. Further research is required to shape future guidance.
Keywords: Axillary clearance; Breast cancer; OSNA; Sentinel lymph node; Total tumour load.
Figures
References
-
- Pegolo E, Puppin C, Gerometta Aet al. . One-step nucleic acid amplification (OSNA) for intraoperative evaluation of sentinel lymph node status in breast cancer: a comparative study between CK19 protein expression and CK19 mRNA level in primary tumors and lymph node metastasis. Virchows Arch 2013; 463: 7–15.10.1007/s00428-013-1440-2 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical