SARS-CoV-2 receptor binding domain fusion protein efficiently neutralizes virus infection
- PMID: 34929007
- PMCID: PMC8722722
- DOI: 10.1371/journal.ppat.1010175
SARS-CoV-2 receptor binding domain fusion protein efficiently neutralizes virus infection
Abstract
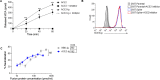
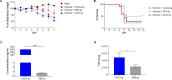
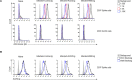
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the COVID-19 pandemic. Currently, as dangerous mutations emerge, there is an increased demand for specific treatments for SARS-CoV-2 infected patients. The spike glycoprotein on the virus envelope binds to the angiotensin converting enzyme 2 (ACE2) on host cells through its receptor binding domain (RBD) to mediate virus entry. Thus, blocking this interaction may inhibit viral entry and consequently stop infection. Here, we generated fusion proteins composed of the extracellular portions of ACE2 and RBD fused to the Fc portion of human IgG1 (ACE2-Ig and RBD-Ig, respectively). We demonstrate that ACE2-Ig is enzymatically active and that it can be recognized by the SARS-CoV-2 RBD, independently of its enzymatic activity. We further show that RBD-Ig efficiently inhibits in-vivo SARS-CoV-2 infection better than ACE2-Ig. Mechanistically, we show that anti-spike antibody generation, ACE2 enzymatic activity, and ACE2 surface expression were not affected by RBD-Ig. Finally, we show that RBD-Ig is more efficient than ACE2-Ig at neutralizing high virus titers. We thus propose that RBD-Ig physically blocks virus infection by binding to ACE2 and that RBD-Ig should be used for the treatment of SARS-CoV-2-infected patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Administration D. Pfizer COVID-19 Vaccine EUA Letter of Authorization reissued 12-23-20. 2020.
-
- Administration D. Moderna COVID-19 Vaccine EUA Letter of Authorization. 2020.
-
- A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants—Full Text View—ClinicalTrials.gov. [cited 6 Apr 2021]. Available: https://clinicaltrials.gov/ct2/show/NCT04505722#wrapper
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

