Long-Term Outcomes After Melody Transcatheter Pulmonary Valve Replacement in the US Investigational Device Exemption Trial
- PMID: 34930015
- PMCID: PMC8765216
- DOI: 10.1161/CIRCINTERVENTIONS.121.010852
Long-Term Outcomes After Melody Transcatheter Pulmonary Valve Replacement in the US Investigational Device Exemption Trial
Abstract
Background: The Melody valve was developed to extend the useful life of previously implanted right ventricular outflow tract (RVOT) conduits or bioprosthetic pulmonary valves, while preserving RV function and reducing the lifetime burden of surgery for patients with complex congenital heart disease.
Methods: Enrollment for the US Investigational Device Exemption study of the Melody valve began in 2007. Extended follow-up was completed in 2020. The primary outcome was freedom from transcatheter pulmonary valve (TPV) dysfunction (freedom from reoperation, reintervention, moderate or severe pulmonary regurgitation, and/or mean RVOT gradient >40 mm Hg). Secondary end points included stent fracture, catheter reintervention, surgical conduit replacement, and death.
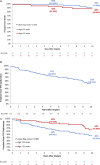
Results: One hundred seventy-one subjects with RVOT conduit or bioprosthetic pulmonary valve dysfunction were enrolled. One hundred fifty underwent Melody TPV replacement. Median age was 19 years (Q1-Q3: 15-26). Median discharge mean RVOT Doppler gradient was 17 mm Hg (Q1-Q3: 12-22). The 149 patients implanted >24 hours were followed for a median of 8.4 years (Q1-Q3: 5.4-10.1). At 10 years, estimated freedom from mortality was 90%, from reoperation 79%, and from any reintervention 60%. Ten-year freedom from TPV dysfunction was 53% and was significantly shorter in children than in adults. Estimated freedom from TPV-related endocarditis was 81% at 10 years (95% CI, 69%-89%), with an annualized rate of 2.0% per patient-year.
Conclusions: Ten-year outcomes from the Melody Investigational Device Exemption trial affirm the benefits of Melody TPV replacement in the lifetime management of patients with RVOT conduits and bioprosthetic pulmonary valves by providing sustained symptomatic and hemodynamic improvement in the majority of patients. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT00740870.
Keywords: endocarditis; heart diseases; humans; mortality; pulmonary valve.
Figures


References
-
- Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0 - PubMed
-
- Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the Melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit: early results from the U.S. Clinical trial. J Am Coll Cardiol. 2009;54:1722–1729. doi: 10.1016/j.jacc.2009.06.034 - PubMed
-
- McElhinney DB, Cheatham JP, Jones TK, Lock JE, Vincent JA, Zahn EM, Hellenbrand WE. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circ Cardiovasc Interv. 2011;4:602–614. doi: 10.1161/CIRCINTERVENTIONS.111.965616 - PubMed
-
- Batra AS, McElhinney DB, Wang W, Zakheim R, Garofano RP, Daniels C, Yung D, Cooper DM, Rhodes J. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J. 2012;163:280–287. doi: 10.1016/j.ahj.2011.10.017 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

