TDP-43 Pathology in Alzheimer's Disease
- PMID: 34930382
- PMCID: PMC8691026
- DOI: 10.1186/s13024-021-00503-x
TDP-43 Pathology in Alzheimer's Disease
Abstract
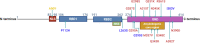
Transactive response DNA binding protein of 43 kDa (TDP-43) is an intranuclear protein encoded by the TARDBP gene that is involved in RNA splicing, trafficking, stabilization, and thus, the regulation of gene expression. Cytoplasmic inclusion bodies containing phosphorylated and truncated forms of TDP-43 are hallmarks of amyotrophic lateral sclerosis (ALS) and a subset of frontotemporal lobar degeneration (FTLD). Additionally, TDP-43 inclusions have been found in up to 57% of Alzheimer's disease (AD) cases, most often in a limbic distribution, with or without hippocampal sclerosis. In some cases, TDP-43 deposits are also found in neurons with neurofibrillary tangles. AD patients with TDP-43 pathology have increased severity of cognitive impairment compared to those without TDP-43 pathology. Furthermore, the most common genetic risk factor for AD, apolipoprotein E4 (APOE4), is associated with increased frequency of TDP-43 pathology. These findings provide strong evidence that TDP-43 pathology is an integral part of multiple neurodegenerative conditions, including AD. Here, we review the biology and pathobiology of TDP-43 with a focus on its role in AD. We emphasize the need for studies on the mechanisms that lead to TDP-43 pathology, especially in the setting of age-related disorders such as AD.
Keywords: Alzheimer’s disease; TARDBP; TDP-43.
© 2021. The Author(s).
Conflict of interest statement
All the authors declare no competing interests.
Figures



References
-
- Ghiso J, Frangione B. Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia. J Alzheimers Dis. 2001;3:65–73. - PubMed
-
- Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–433. - PubMed
-
- Spillantini MG, Murrell JR, Goedert M, Farlow M, Klug A, Ghetti B. Mutations in the tau gene (MAPT) in FTDP-17: the family with Multiple System Tauopathy with Presenile Dementia (MSTD) J Alzheimers Dis. 2006;9:373–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

