Cytoglobin has potent superoxide dismutase function
- PMID: 34930834
- PMCID: PMC8719900
- DOI: 10.1073/pnas.2105053118
Cytoglobin has potent superoxide dismutase function
Abstract
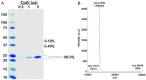
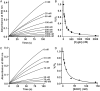
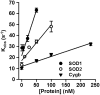
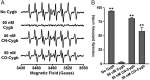
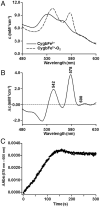
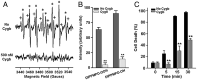
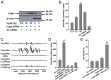
Cytoglobin (Cygb) was discovered as a novel type of globin that is expressed in mammals; however, its functions remain uncertain. While Cygb protects against oxidant stress, the basis for this is unclear, and the effect of Cygb on superoxide metabolism is unknown. From dose-dependent studies of the effect of Cygb on superoxide catabolism, we identify that Cygb has potent superoxide dismutase (SOD) function. Initial assays using cytochrome c showed that Cygb exhibits a high rate of superoxide dismutation on the order of 108 M-1 ⋅ s-1 Spin-trapping studies also demonstrated that the rate of Cygb-mediated superoxide dismutation (1.6 × 108 M-1 ⋅ s-1) was only ∼10-fold less than Cu,Zn-SOD. Stopped-flow experiments confirmed that Cygb rapidly dismutates superoxide with rates within an order of magnitude of Cu,Zn-SOD or Mn-SOD. The SOD function of Cygb was inhibited by cyanide and CO that coordinate to Fe3+-Cygb and Fe2+-Cygb, respectively, suggesting that dismutation involves iron redox cycling, and this was confirmed by spectrophotometric titrations. In control smooth-muscle cells and cells with siRNA-mediated Cygb knockdown subjected to extracellular superoxide stress from xanthine/xanthine oxidase or intracellular superoxide stress triggered by the uncoupler, menadione, Cygb had a prominent role in superoxide metabolism and protected against superoxide-mediated death. Similar experiments in vessels showed higher levels of superoxide in Cygb-/- mice than wild type. Thus, Cygb has potent SOD function and can rapidly dismutate superoxide in cells, conferring protection against oxidant injury. In view of its ubiquitous cellular expression at micromolar concentrations in smooth-muscle and other cells, Cygb can play an important role in cellular superoxide metabolism.
Keywords: EPR; ROS; SOD; free radical; superoxide.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Burmester T., Ebner B., Weich B., Hankeln T., Cytoglobin: A novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 19, 416–421 (2002). - PubMed
-
- Kawada N., et al. , Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J. Biol. Chem. 276, 25318–25323 (2001). - PubMed
-
- Trent J. T. III, Hargrove M. S., A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 277, 19538–19545 (2002). - PubMed
-
- Hankeln T., et al. , Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J. Inorg. Biochem. 99, 110–119 (2005). - PubMed
Publication types
MeSH terms
Substances
Associated data
- figshare/10.6084/m9.figshare.14802927
- figshare/10.6084/m9.figshare.16918909
- figshare/10.6084/m9.figshare.16918969
- figshare/10.6084/m9.figshare.16919011
- figshare/10.6084/m9.figshare.16919014
- figshare/10.6084/m9.figshare.16919026
- figshare/10.6084/m9.figshare.16919047
- figshare/10.6084/m9.figshare.16919068
- figshare/10.6084/m9.figshare.16919074
- figshare/10.6084/m9.figshare.16919086
- figshare/10.6084/m9.figshare.16919182
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

