Contributions of NaV1.8 and NaV1.9 to excitability in human induced pluripotent stem-cell derived somatosensory neurons
- PMID: 34930944
- PMCID: PMC8688473
- DOI: 10.1038/s41598-021-03608-x
Contributions of NaV1.8 and NaV1.9 to excitability in human induced pluripotent stem-cell derived somatosensory neurons
Abstract
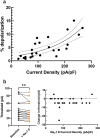
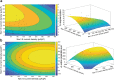
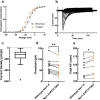
The inhibition of voltage-gated sodium (NaV) channels in somatosensory neurons presents a promising novel modality for the treatment of pain. However, the precise contribution of these channels to neuronal excitability, the cellular correlate of pain, is unknown; previous studies using genetic knockout models or pharmacologic block of NaV channels have identified general roles for distinct sodium channel isoforms, but have never quantified their exact contributions to these processes. To address this deficit, we have utilized dynamic clamp electrophysiology to precisely tune in varying levels of NaV1.8 and NaV1.9 currents into induced pluripotent stem cell-derived sensory neurons (iPSC-SNs), allowing us to quantify how graded changes in these currents affect different parameters of neuronal excitability and electrogenesis. We quantify and report direct relationships between NaV1.8 current density and action potential half-width, overshoot, and repetitive firing. We additionally quantify the effect varying NaV1.9 current densities have on neuronal membrane potential and rheobase. Furthermore, we examined the simultaneous interplay between NaV1.8 and NaV1.9 on neuronal excitability. Finally, we show that minor biophysical changes in the gating of NaV1.8 can render human iPSC-SNs hyperexcitable, in a first-of-its-kind investigation of a gain-of-function NaV1.8 mutation in a human neuronal background.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Raftery MN, et al. Chronic pain in the Republic of Ireland–community prevalence, psychosocial profile and predictors of pain-related disability: Results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain. 2011;152:1096–1103. doi: 10.1016/j.pain.2011.01.019. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

