Long-term macrolide treatment for non-cystic fibrosis bronchiectasis in children: a meta-analysis
- PMID: 34930997
- PMCID: PMC8688433
- DOI: 10.1038/s41598-021-03778-8
Long-term macrolide treatment for non-cystic fibrosis bronchiectasis in children: a meta-analysis
Abstract
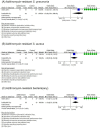
Recurrent bacterial infection causes frequent bronchiectasis (BE) exacerbations. The effectiveness and safety of long-term administration of macrolides in BE remain controversial, especially in children who require minimal treatment to prevent exacerbation. We conducted this meta-analysis to determine the usefulness of long-term macrolide use in pediatric BE. We searched PubMed, Cochrane Library databases, Embase, KoreaMed, Igaku Chuo Zasshi, and Chinese National Knowledge Infrastructure databases. We identified randomized controlled trials (RCTs) which elucidated long-term macrolide treatment (≥ 4 weeks) in non-cystic fibrosis BE in children aged < 18 years. The primary outcome was frequency of acute exacerbation; secondary outcomes included changes in pulmonary function, sputum scores, and adverse events including bacterial resistance. We included four RCTs. Long-term macrolide treatment showed a significant decrease in the frequency of exacerbation (odds ratio [OR], 0.30; 95% confidence interval [CI], 0.10-0.87), mean number of exacerbations per patient (mean difference, - 1.40; 95% CI, - 2.26 to - 0.54), and sputum purulence score (mean difference, - 0.78; 95% CI, - 1.32 to - 0.24). However, long-term macrolide treatment was accompanied by an increased carriage of azithromycin-resistant bacteria (OR, 7.13). Long-term macrolide administration prevents exacerbation of BE in children; however, there are risks of increasing antibiotic resistance. Benefits and risks should be weighed and determined on a patient-by-patient basis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



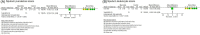


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

