Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue
- PMID: 34931077
- PMCID: PMC9207157
- DOI: 10.1038/s41551-021-00815-9
Genetically engineered and enucleated human mesenchymal stromal cells for the targeted delivery of therapeutics to diseased tissue
Abstract
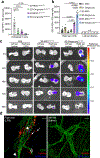
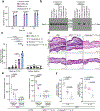
Targeting the delivery of therapeutics specifically to diseased tissue enhances their efficacy and decreases their side effects. Here we show that mesenchymal stromal cells with their nuclei removed by density-gradient centrifugation following the genetic modification of the cells for their display of chemoattractant receptors and endothelial-cell-binding molecules are effective vehicles for the targeted delivery of therapeutics. The enucleated cells neither proliferate nor permanently engraft in the host, yet retain the organelles for energy and protein production, undergo integrin-regulated adhesion to inflamed endothelial cells, and actively home to chemokine gradients established by diseased tissues. In mouse models of acute inflammation and of pancreatitis, systemically administered enucleated cells expressing two types of chemokine receptor and an endothelial adhesion molecule enhanced the delivery of an anti-inflammatory cytokine to diseased tissue (with respect to unmodified stromal cells and to exosomes derived from bone-marrow-derived stromal cells), attenuating inflammation and ameliorating disease pathology. Enucleated cells retain most of the cells' functionality, yet acquire the cargo-carrying characteristics of cell-free delivery systems, and hence represent a versatile delivery vehicle and therapeutic system.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest
Cargocyte is the trademark of Cytonus Therapeutics. R.L.K is the co-founder and equity holder of Cytonus Therapeutics. H.W and R.L.K are co-inventors of a related patent (WO 2019/032628 A1, US 10,927,349 B2) and H.W., W.P., and R.L.K. are co-inventors of another related patent (PCT/US21/016,919). Both patents were filed by UCSD.
Figures




References
-
- Allen TM & Cullis PR Drug delivery systems: entering the mainstream. Science 303, 1818–1822 (2004). - PubMed
-
- Yoo JW, Irvine DJ, Discher DE & Mitragotri S Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov 10, 521–535 (2011). - PubMed
-
- Lyerly HK, Osada T & Hartman ZC Right Time and Place for IL12: Targeted Delivery Stimulates Immune Therapy. Clin Cancer Res 25, 9–11 (2019). - PubMed
-
- Fioranelli M & Roccia MG Twenty-five years of studies and trials for the therapeutic application of IL-10 immunomodulating properties. From high doses administration to low dose medicine new paradigm. J Integr Cardiol 1, 2–6 (2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

