Preventive Vaccination with Mesenchymal Stem Cells Protects Mice from Lethal Infection Caused by Herpes Simplex Virus 1
- PMID: 34931092
- PMCID: PMC8675305
- DOI: 10.1134/S0026893321020242
Preventive Vaccination with Mesenchymal Stem Cells Protects Mice from Lethal Infection Caused by Herpes Simplex Virus 1
Abstract
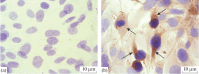
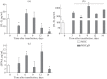
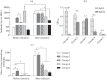
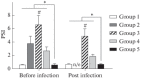
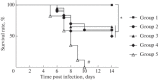
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) infect almost all organs and tissues, cause genital herpes-the most common sexually transmitted disease-disorders of the central nervous system (CNS), and lead to severe complications in children. Despite the available drugs, the incidence of HSV-1/2 continues to rise. None of the prophylactic vaccine candidates have shown a protective effect in trials nor approval for use in clinical practice. We have investigated the protective properties of mesenchymal stem cells (MSC) isolated from the bone marrow of mice. A comparative analysis of the protective response to the introduction of primary and modified MSCs (mMSC) was carried out using the plasmid containing gene of the HSV and an inactivated virus in a model of lethal HSV-1 infection in mice. mMSCs were obtained by transfection of the Us6 gene encoding glycoprotein D (gD) of the HSV, the plasmid contained the same gene. After twofold immunization with primary MSCs, the formation of antibodies interacting with the viral antigen (according to enzyme immunoassay data) and neutralizing the infectious activity of HSV-1 in the reaction of biological neutralization was observed in the peripheral blood of mice. In addition, the introduction of primary MSCs induced the production of interferon gamma (INF-γ) which is detected in the peripheral blood of mice. After infection with HSV-1, the immunized mice showed significantly increased titers of virus-specific antibodies, an increased level of IFNγ, and were completely protected from lethal HSV-1 infection. The protective effect of the other three immunogens was lower and did not exceed 50-65%. Considering the wide availability of MSCs, the proven safety of intravenous administration, and the results obtained in this work on the ability to induce innate, adaptive and protective immunity to HSV-1, MSCs can be considered a promising basis for the development of new cellular vaccines for the prevention of herpesvirus and other viral infections.
Keywords: DNA immunization; genetically modified mesenchymal stem cells; herpes simplex virus 1; mesenchymal stem cells; protective activity; recombinant DNA; transfection.
© Pleiades Publishing, Inc. 2021, ISSN 0026-8933, Molecular Biology, 2021, Vol. 55, No. 3, pp. 413–423. © Pleiades Publishing, Inc., 2021.Russian Text © The Author(s), 2021, published in Molekulyarnaya Biologiya, 2021, Vol. 55, No. 3, pp. 478–490.
Conflict of interest statement
Conflict of interest. The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
[Preventive Vaccination with Mesenchymal Stem Cells Protects Mice from Lethal Infection Caused by Herpes Simplex Virus 1].Mol Biol (Mosk). 2021 May-Jun;55(3):478-490. doi: 10.31857/S0026898421030101. Mol Biol (Mosk). 2021. PMID: 34097682 Russian.
-
Vaccination with the Secreted Glycoprotein G of Herpes Simplex Virus 2 Induces Protective Immunity after Genital Infection.Viruses. 2016 Apr 22;8(4):110. doi: 10.3390/v8040110. Viruses. 2016. PMID: 27110813 Free PMC article.
-
CCL19 and CCL28 Assist Herpes Simplex Virus 2 Glycoprotein D To Induce Protective Systemic Immunity against Genital Viral Challenge.mSphere. 2021 Apr 28;6(2):e00058-21. doi: 10.1128/mSphere.00058-21. mSphere. 2021. PMID: 33910988 Free PMC article.
-
Immune responses to herpes simplex virus in guinea pigs (footpad model) and mice immunized with vaccinia virus recombinants containing herpes simplex virus glycoprotein D.Rev Infect Dis. 1991 Nov-Dec;13 Suppl 11:S924-34. doi: 10.1093/clind/13.supplement_11.s924. Rev Infect Dis. 1991. PMID: 1664130 Review.
-
Live vaccinia virus recombinants expressing herpes simplex virus genes.Rev Infect Dis. 1991 Nov-Dec;13 Suppl 11:S898-903. doi: 10.1093/clind/13.supplement_11.s898. Rev Infect Dis. 1991. PMID: 1664124 Review.
Cited by
-
Human Mesenchymal Stem Cells Modified with the NS5A Gene of Hepatitis C Virus Induce a Cellular Immune Response Exceeding the Response to DNA Immunization with This Gene.Biology (Basel). 2023 May 30;12(6):792. doi: 10.3390/biology12060792. Biology (Basel). 2023. PMID: 37372076 Free PMC article.
-
Recovery from Bell's palsy after treatment using uncultured umbilical cord-derived mesenchymal stem cells: A case report.World J Clin Cases. 2023 Apr 26;11(12):2817-2824. doi: 10.12998/wjcc.v11.i12.2817. World J Clin Cases. 2023. PMID: 37214571 Free PMC article.
References
-
- Gottlieb S.L., Giersing B., Boily M.C., Chesson H., Looker K.J., Schiffer J., Spicknall I., Hutubessy R., Broutet N., WHO HSV Vaccine Impact Modelling Meeting Working Group Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: key findings from the World Health Organization consultation on HSV vaccine impact modelling. Vaccine. 2019;37:7336–7345. doi: 10.1016/j.vaccine.2017.03.074. - DOI - PMC - PubMed
-
- Looker K.J., Welton N.J., Sabin K.M., Dalal S., Vickerman P, Turner K.M.E., Boily M.C., Gottlieb S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020;20:240–249. doi: 10.1016/S1473-3099(19)30470-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
