Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia
- PMID: 34931176
- PMCID: PMC8673819
- DOI: 10.1016/j.bbrep.2021.101187
Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia
Abstract
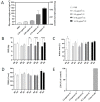
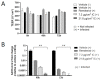
Iota-carrageenan (IC) nasal spray, a medical device approved for treating respiratory viral infections, has previously been shown to inhibit the ability of a variety of respiratory viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), to enter and replicate in the cell by interfering with the virus binding to the cell surface. The aim of this study was to further investigate the efficacy and safety of IC in SARS-CoV-2 infection in advanced in vitro models of the human respiratory epithelium, the primary target and entry port for SARS-CoV-2. We extended the in vitro safety assessment of nebulized IC in a 3-dimensional model of reconstituted human bronchial epithelium, and we demonstrated the efficacy of IC in protecting reconstituted nasal epithelium against viral infection and replication of a patient-derived SARS-CoV-2 strain. The results obtained from these two advanced models of human respiratory tract epithelia confirm previous findings from in vitro SARS-CoV-2 infection assays and demonstrate that topically applied IC can effectively prevent SARS-CoV-2 infection and replication. Moreover, the absence of toxicity and functional and structural impairment of the mucociliary epithelium demonstrates that the nebulized IC is well tolerated.
Keywords: 3D, 3-dimensional; AE, after exposure; ALI, air–liquid interface; Air–liquid interface; BE, before exposure; Bronchial epithelium; CBF, ciliary beating frequency; COVID-19; COVID19, Coronavirus disease 2019; DMMB, Dimethylmethylene blue; IC, Iota-carrageenan; Iota-carrageenan; LDH, lactate dehydrogenase; MOI, multiplicity of infection; NHBE, normal human bronchial epithelial; Nasal epithelium; Nasal spray; PBS, phosphate-buffered saline; SARS-CoV-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SSPL, spike-pseudotyped lentivirus; TEER, transepithelial electrical resistance; hACE2, human angiotensin I-converting enzyme 2.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro.PLoS One. 2021 Feb 17;16(2):e0237480. doi: 10.1371/journal.pone.0237480. eCollection 2021. PLoS One. 2021. PMID: 33596218 Free PMC article.
-
ColdZyme Maintains Integrity in SARS-CoV-2-Infected Airway Epithelia.mBio. 2021 Apr 27;12(2):e00904-21. doi: 10.1128/mBio.00904-21. mBio. 2021. PMID: 33906927 Free PMC article.
-
Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia.Int J Mol Sci. 2022 Apr 6;23(7):4062. doi: 10.3390/ijms23074062. Int J Mol Sci. 2022. PMID: 35409423 Free PMC article.
-
The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: A critical review.Clin Epidemiol Glob Health. 2021 Oct-Dec;12:100826. doi: 10.1016/j.cegh.2021.100826. Epub 2021 Jun 29. Clin Epidemiol Glob Health. 2021. PMID: 34222718 Free PMC article. Review.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
Cited by
-
Improving Nasal Protection for Preventing SARS-CoV-2 Infection.Biomedicines. 2022 Nov 17;10(11):2966. doi: 10.3390/biomedicines10112966. Biomedicines. 2022. PMID: 36428534 Free PMC article.
-
Carrageenans and the Carrageenan-Echinochrome Complex as Anti-SARS-CoV-2 Agents.Int J Mol Sci. 2025 Jun 26;26(13):6175. doi: 10.3390/ijms26136175. Int J Mol Sci. 2025. PMID: 40649954 Free PMC article.
-
Edible algae (Ecklonia cava) bioprocessed with mycelia of shiitake (Lentinula edodes) mushrooms in liquid culture and its isolated fractions protect mice against allergic asthma.BMC Complement Med Ther. 2022 Sep 17;22(1):242. doi: 10.1186/s12906-022-03705-y. BMC Complement Med Ther. 2022. PMID: 36115955 Free PMC article.
-
Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection.Int J Mol Sci. 2024 Apr 9;25(8):4142. doi: 10.3390/ijms25084142. Int J Mol Sci. 2024. PMID: 38673729 Free PMC article.
-
Over-the-counter carrageenan-based sprays may interfere with PCR testing of nasopharyngeal swabs to detect SARS-CoV-2.PLoS One. 2025 Feb 6;20(2):e0316700. doi: 10.1371/journal.pone.0316700. eCollection 2025. PLoS One. 2025. PMID: 39913533 Free PMC article.
References
-
- ECDC COVID-19 situation update worldwide. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases as of week 23, updated 17 June 2021. 2021 [cited 2021 june 24, 2021]; Available from:
-
- Sanders J.M., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020;323(18):1824–1836. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

