This is a preprint.
Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy
- PMID: 34931183
- PMCID: PMC8687466
- DOI: 10.21203/rs.3.rs-1150427/v1
Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and early infancy
Update in
-
Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy.Nat Commun. 2022 Jul 30;13(1):4422. doi: 10.1038/s41467-022-32188-1. Nat Commun. 2022. PMID: 35908075 Free PMC article.
Abstract
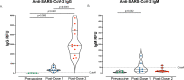
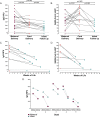
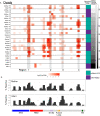
Studies are needed to evaluate the safety and effectiveness of mRNA SARS-CoV-2 vaccination during pregnancy, and the levels of protection provided to their newborns through placental transfer of antibodies. We evaluated the transplacental transfer of mRNA vaccine products and functional anti-SARS-CoV-2 antibodies during pregnancy and early infancy in a cohort of 20 individuals vaccinated during pregnancy. We found no evidence of mRNA vaccine products in maternal blood, placenta tissue, or cord blood at delivery. However, we found time-dependent efficient transfer of IgG and neutralizing antibodies to the neonate that persisted during early infancy. Additionally, using phage immunoprecipitation sequencing, we found a vaccine-specific signature of SARS-CoV-2 Spike protein epitope binding that is transplacentally transferred during pregnancy. In conclusion, products of mRNA vaccines are not transferred to the fetus during pregnancy, however timing of vaccination during pregnancy is critical to ensure transplacental transfer of protective antibodies during early infancy.
Figures


References
-
- CDC. COVID-19 vaccination among pregnant people aged 18–49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,* United States, <https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women> (2021).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

