This is a preprint.
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling
- PMID: 34931188
- PMCID: PMC8687463
- DOI: 10.1101/2021.12.10.472112
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling
Update in
-
SARS-CoV-2 Spike triggers barrier dysfunction and vascular leak via integrins and TGF-β signaling.Nat Commun. 2022 Dec 9;13(1):7630. doi: 10.1038/s41467-022-34910-5. Nat Commun. 2022. PMID: 36494335 Free PMC article.
Abstract
Severe COVID-19 is associated with epithelial and endothelial barrier dysfunction within the lung as well as in distal organs. While it is appreciated that an exaggerated inflammatory response is associated with barrier dysfunction, the triggers of this pathology are unclear. Here, we report that cell-intrinsic interactions between the Spike (S) glycoprotein of SARS-CoV-2 and epithelial/endothelial cells are sufficient to trigger barrier dysfunction in vitro and vascular leak in vivo , independently of viral replication and the ACE2 receptor. We identify an S-triggered transcriptional response associated with extracellular matrix reorganization and TGF-β signaling. Using genetic knockouts and specific inhibitors, we demonstrate that glycosaminoglycans, integrins, and the TGF-β signaling axis are required for S-mediated barrier dysfunction. Our findings suggest that S interactions with barrier cells are a contributing factor to COVID-19 disease severity and offer mechanistic insight into SARS-CoV-2 triggered vascular leak, providing a starting point for development of therapies targeting COVID-19 pathogenesis.
Conflict of interest statement
Declarations of Interest
The authors declare no competing interests.
Figures

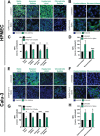

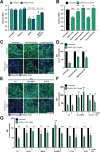
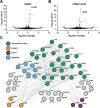
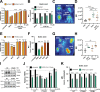
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
