This is a preprint.
SARS-CoV2 variant-specific replicating RNA vaccines protect from disease and pathology and reduce viral shedding following challenge with heterologous SARS-CoV2 variants of concern
- PMID: 34931189
- PMCID: PMC8687464
- DOI: 10.1101/2021.12.10.472134
SARS-CoV2 variant-specific replicating RNA vaccines protect from disease and pathology and reduce viral shedding following challenge with heterologous SARS-CoV2 variants of concern
Update in
-
SARS-CoV2 variant-specific replicating RNA vaccines protect from disease following challenge with heterologous variants of concern.Elife. 2022 Feb 22;11:e75537. doi: 10.7554/eLife.75537. Elife. 2022. PMID: 35191378 Free PMC article.
Abstract
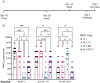
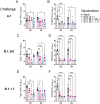
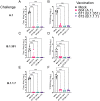
Despite mass public health efforts, the SARS-CoV2 pandemic continues as of late-2021 with resurgent case numbers in many parts of the world. The emergence of SARS-CoV2 variants of concern (VoC) and evidence that existing vaccines that were designed to protect from the original strains of SARS-CoV-2 may have reduced potency for protection from infection against these VoC is driving continued development of second generation vaccines that can protect against multiple VoC. In this report, we evaluated an alphavirus-based replicating RNA vaccine expressing Spike proteins from the original SARS-CoV-2 Alpha strain and recent VoCs delivered in vivo via a lipid inorganic nanoparticle. Vaccination of both mice and Syrian Golden hamsters showed that vaccination induced potent neutralizing titers against each homologous VoC but reduced neutralization against heterologous challenges. Vaccinated hamsters challenged with homologous SARS-CoV2 variants exhibited complete protection from infection. In addition, vaccinated hamsters challenged with heterologous SARS-CoV-2 variants exhibited significantly reduced shedding of infectious virus. Our data demonstrate that this vaccine platform elicits significant protective immunity against SARS-CoV2 variants and supports continued development of this platform.
Conflict of interest statement
Figures




Similar articles
-
SARS-CoV2 variant-specific replicating RNA vaccines protect from disease following challenge with heterologous variants of concern.Elife. 2022 Feb 22;11:e75537. doi: 10.7554/eLife.75537. Elife. 2022. PMID: 35191378 Free PMC article.
-
Mucosal immunization with Ad5-based vaccines protects Syrian hamsters from challenge with omicron and delta variants of SARS-CoV-2.Front Immunol. 2023 Feb 22;14:1086035. doi: 10.3389/fimmu.2023.1086035. eCollection 2023. Front Immunol. 2023. PMID: 36911687 Free PMC article.
-
Replicating RNA platform enables rapid response to the SARS-CoV-2 Omicron variant and elicits enhanced protection in naïve hamsters compared to ancestral vaccine.EBioMedicine. 2022 Sep;83:104196. doi: 10.1016/j.ebiom.2022.104196. Epub 2022 Aug 4. EBioMedicine. 2022. PMID: 35932641 Free PMC article.
-
SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies.Int Rev Immunol. 2023;42(6):393-414. doi: 10.1080/08830185.2022.2079642. Epub 2022 May 28. Int Rev Immunol. 2023. PMID: 35635216 Review.
-
Host Response to SARS-CoV2 and Emerging Variants in Pre-Existing Liver and Gastrointestinal Diseases.Front Cell Infect Microbiol. 2021 Oct 25;11:753249. doi: 10.3389/fcimb.2021.753249. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34760721 Free PMC article. Review.
References
-
- Edara V.-V., Pinsky B.A., Suthar M.S., Lai L., Davis-Gardner M.E., Floyd K., Flowers M.W., Wrammert J., Hussaini L., Ciric C.R., et al. (2021). Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. New England Journal of Medicine 385, 664–666. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
