This is a preprint.
Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization
- PMID: 34931200
- PMCID: PMC8687471
- DOI: 10.1101/2021.12.15.21267805
Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization
Update in
-
SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination.N Engl J Med. 2022 Mar 17;386(11):1088-1091. doi: 10.1056/NEJMc2119912. Epub 2022 Jan 26. N Engl J Med. 2022. PMID: 35081298 Free PMC article. No abstract available.
Abstract
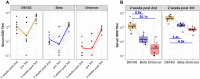
The Omicron variant of SARS-CoV-2 is raising concerns because of its increased transmissibility and potential for reduced susceptibility to antibody neutralization. To assess the potential risk of this variant to existing vaccines, serum samples from mRNA-1273 vaccine recipients were tested for neutralizing activity against Omicron and compared to neutralization titers against D614G and Beta in live virus and pseudovirus assays. Omicron was 41-84-fold less sensitive to neutralization than D614G and 5.3-7.4-fold less sensitive than Beta when assayed with serum samples obtained 4 weeks after 2 standard inoculations with 100 μg mRNA-1273. A 50 μg boost increased Omicron neutralization titers and may substantially reduce the risk of symptomatic vaccine breakthrough infections.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
