Galactose Oxidase Enables Modular Assembly of Conjugates from Native Antibodies with High Drug-to-Antibody Ratios
- PMID: 34931761
- PMCID: PMC9303943
- DOI: 10.1002/cssc.202102592
Galactose Oxidase Enables Modular Assembly of Conjugates from Native Antibodies with High Drug-to-Antibody Ratios
Abstract
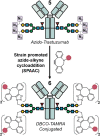
The potential of antibody conjugates with high drug loading in anticancer therapy has recently been highlighted by the approval of Trastuzumab deruxtecan and Sacituzumab govitecan. These biopharmaceutical approaches have spurred interest in bioconjugation strategies with high and defined degrees of drug-to-antibody ratio (DAR), in particular on native antibodies. Here, a glycoengineering methodology was developed to generate antibody drug conjugates with DAR of up to eight, by combining highly selective enzymatic galactosylation and oxidation with biorthogonal tandem Knoevenagel-Michael addition chemistry. This four-step approach offers a selective route to conjugates from native antibodies with high drug loading, and thus illustrates how biocatalysis can be used for the generation of biopharmaceuticals using mild reaction conditions.
Keywords: antibodies; antibody-drug conjugates; biocatalysis; glycoengineering; medicinal chemistry.
© 2021 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

