Surface Properties of Colloidal Particles Affect Colloidal Self-Assembly in Evaporating Self-Lubricating Ternary Droplets
- PMID: 34931807
- PMCID: PMC8763378
- DOI: 10.1021/acsami.1c19241
Surface Properties of Colloidal Particles Affect Colloidal Self-Assembly in Evaporating Self-Lubricating Ternary Droplets
Abstract
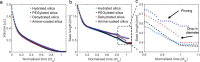
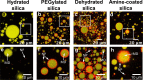
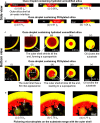
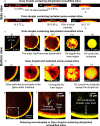
In this work, we unravel the role of surface properties of colloidal particles on the formation of supraparticles (clusters of colloidal particles) in a colloidal Ouzo droplet. Self-lubricating colloidal Ouzo droplets are an efficient and simple approach to form supraparticles, overcoming the challenge of the coffee stain effect in situ. Supraparticles are an efficient route to high-performance materials in various fields, from catalysis to carriers for therapeutics. Yet, the role of the surface of colloidal particles in the formation of supraparticles using Ouzo droplets remains unknown. Therefore, we used silica particles as a model system and compared sterically stabilized versus electrostatically stabilized silica particles─positively and negatively charged. Additionally, we studied the effect of hydration. Hydrated negatively charged silica particles and sterically stabilized silica particles form supraparticles. Conversely, dehydrated negatively charged silica particles and positively charged amine-coated particles form flat film-like deposits. Notably, the assembly process is different for all the four types of particles. The surface modifications alter (a) the contact line motion of the Ouzo droplet and (b) the particle-oil and particle-substrate interactions. These alterations modify the particle accumulation at the various interfaces, which ultimately determines the shape of the final deposit. Thus, by modulating the surface properties of the colloidal particles, we can tune the shape of the final deposit, from a spheroidal supraparticle to a flat deposit. In the future, this approach can be used to tailor the supraparticles for applications such as optics and catalysis, where the shape affects the functionality.
Keywords: Ouzo effect; colloidal self-assembly; colloidal stabilization; evaporation induced self-assembly; self-lubrication; silica particles; supraparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Particle Size Determines the Shape of Supraparticles in Self-Lubricating Ternary Droplets.ACS Nano. 2021 Mar 23;15(3):4256-4267. doi: 10.1021/acsnano.0c06814. Epub 2021 Feb 19. ACS Nano. 2021. PMID: 33601887 Free PMC article.
-
Porous supraparticle assembly through self-lubricating evaporating colloidal ouzo drops.Nat Commun. 2019 Jan 29;10(1):478. doi: 10.1038/s41467-019-08385-w. Nat Commun. 2019. PMID: 30696829 Free PMC article.
-
Evaporation-driven Supraparticle Synthesis by Self-Lubricating Colloidal Dispersion Microdrops.ACS Appl Mater Interfaces. 2023 Aug 16;15(32):38986-38995. doi: 10.1021/acsami.3c07719. Epub 2023 Aug 2. ACS Appl Mater Interfaces. 2023. PMID: 37530444
-
Droplet evaporation on super liquid-repellent surfaces: A controllable approach for supraparticle fabrication.Adv Colloid Interface Sci. 2024 Dec;334:103305. doi: 10.1016/j.cis.2024.103305. Epub 2024 Sep 30. Adv Colloid Interface Sci. 2024. PMID: 39388856 Review.
-
Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials.Gels. 2017 May 4;3(2):15. doi: 10.3390/gels3020015. Gels. 2017. PMID: 30920512 Free PMC article. Review.
Cited by
-
Coacervate-mediated novel pancreatic cancer drug Aleuria Aurantia lectin delivery for augmented anticancer therapy.Biomater Res. 2022 Jul 22;26(1):35. doi: 10.1186/s40824-022-00282-6. Biomater Res. 2022. PMID: 35869562 Free PMC article.
-
Self-organization of agitated microspheres on various substrates.Soft Matter. 2022 May 18;18(19):3660-3677. doi: 10.1039/d2sm00432a. Soft Matter. 2022. PMID: 35485633 Free PMC article.
References
-
- Liu J.; Xiao M.; Li C.; Li H.; Wu Z.; Zhu Q.; Tang R.; Xu A. B.; He L. Rugby-Ball-Like Photonic Crystal Supraparticles with Non-Close-Packed Structures and Multiple Magneto-Optical Responses. J. Mater. Chem. C 2019, 7 (47), 15042–15048. 10.1039/C9TC05438C. - DOI
LinkOut - more resources
Full Text Sources

