Study of Treatment and Reproductive Outcomes Among Reproductive-Age Women With HIV Infection in the Southern United States: Protocol for a Longitudinal Cohort Study
- PMID: 34932006
- PMCID: PMC8726043
- DOI: 10.2196/30398
Study of Treatment and Reproductive Outcomes Among Reproductive-Age Women With HIV Infection in the Southern United States: Protocol for a Longitudinal Cohort Study
Abstract
Background: Nearly a quarter of the 1.1 million individuals with HIV in the United States are women. Racial and ethnic minority women in the Southern United States are disproportionately impacted. Reproductive-age women with HIV are prone to poor HIV outcomes but remain underrepresented in HIV research. We will answer contemporary questions related to the health outcomes in this population by enrolling a prospective cohort of reproductive-age women with and without HIV in the Southern United States.
Objective: The Study of Treatment and Reproductive Outcomes (STAR) will enroll and retain 2000 reproductive-age women with and without HIV. The STAR will leverage the infrastructure of the US-based Multicenter AIDS Cohort Study (MACS)/Women's Interagency HIV Study (WIHS) Combined Cohort Study, comprising the WIHS (a cohort of women with and at risk for HIV, which began in 1993), and the MACS (a cohort of gay and bisexual men with and at risk for HIV, which began in 1984). Although the advancing age of the participants enrolled in the MACS/WIHS Combined Cohort Study provides an opportunity to address the questions related to HIV and aging, the research questions pertinent to the reproductive years must also be addressed. The STAR will conduct high-priority scientific research in key areas with the overall aim of addressing the unique needs of reproductive-age women with HIV.
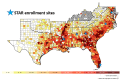
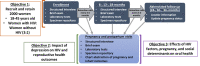
Methods: The STAR is a prospective, observational cohort study that will be conducted at 6 sites in the United States-Atlanta, Georgia; Birmingham, Alabama; Jackson, Mississippi; Chapel Hill, North Carolina; Miami, Florida; and Washington, District of Columbia. Visits will occur semiannually for 2 years, with additional visits for up to 5 years. At each visit, the participating women will complete a structured interview for collecting key demographic, psychosocial, and clinical variables, and undergo biospecimen collection for laboratory testing and repositing (blood, urine, hair, vaginal, anal, and oral specimens). Pregnant women and infants will undergo additional study assessments. The initial scientific focus of the STAR is to understand the roles of key social determinants of health, depression, reproductive health, and oral health on HIV and pregnancy outcomes across the reproductive life span.
Results: Enrollment in the STAR commenced in February 2021 and is ongoing.
Conclusions: Through in-depth, longitudinal data and biospecimen collection, the newly initiated STAR cohort will create a platform to answer scientific questions regarding reproductive-age women with and without HIV. STAR will be uniquely positioned to enable investigators to conduct high-impact research relevant to this population. Building on the legacy of the MACS and WIHS cohorts, the STAR is designed to foster multidisciplinary collaborations to galvanize scientific discoveries to improve the health of reproductive-age women with HIV and ameliorate the effects of the HIV epidemic in this population in the United States.
Keywords: HIV; depression; longitudinal cohort study; oral health; women’s health.
©Anandi N Sheth, Adaora A Adimora, Elizabeth Topper Golub, Seble G Kassaye, Aadia Rana, Daniel Westreich, Jennifer Webster Cyriaque, Carrigan Parish, Deborah Konkle-Parker, Deborah L Jones, Mirjam-Colette Kempf, Igho Ofotokun, Ruth M Kanthula, Jessica Donohue, Patricia Raccamarich, Tina Tisdale, Catalina Ramirez, Lari Warren-Jeanpiere, Phyllis C Tien, Maria L Alcaide. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 20.12.2021.
Conflict of interest statement
Conflicts of Interest: DW has engaged in consulting for PRI Healthcare Solutions on behalf of Sanofi Pasteur on subjects unrelated to this work. MLA receives honorarium from the following sources (unrelated to this work): Merck Inc (educational lecture fees) and Senhwa pharmaceuticals (Data Safety Monitoring Board member). AAA has received consulting fees and funds for research to her institution from Merck and Gilead.
Figures
Similar articles
-
Characteristics of the MACS/WIHS Combined Cohort Study: Opportunities for Research on Aging With HIV in the Longest US Observational Study of HIV.Am J Epidemiol. 2021 Aug 1;190(8):1457-1475. doi: 10.1093/aje/kwab050. Am J Epidemiol. 2021. PMID: 33675224 Free PMC article.
-
American Cohort to Study HIV Acquisition Among Transgender Women in High-Risk Areas (The LITE Study): Protocol for a Multisite Prospective Cohort Study in the Eastern and Southern United States.JMIR Res Protoc. 2019 Oct 3;8(10):e14704. doi: 10.2196/14704. JMIR Res Protoc. 2019. PMID: 31584005 Free PMC article.
-
Digital Epidemiologic Research on Multilevel Risks for HIV Acquisition and Other Health Outcomes Among Transgender Women in Eastern and Southern United States: Protocol for an Online Cohort.JMIR Res Protoc. 2021 Apr 26;10(4):e29152. doi: 10.2196/29152. JMIR Res Protoc. 2021. PMID: 33900202 Free PMC article.
-
HIV/AIDS: a minority health issue.Med Clin North Am. 2005 Jul;89(4):895-912. doi: 10.1016/j.mcna.2005.03.005. Med Clin North Am. 2005. PMID: 15925655 Review.
-
Understanding U.S. fertility: continuity and change in the National Survey of Family Growth, 1988-1995.Fam Plann Perspect. 1996 Jan-Feb;28(1):4-12. Fam Plann Perspect. 1996. PMID: 8822409 Review.
Cited by
-
Attitudes Towards Aging, Depression, Physical Functioning, and Pain Among Women Living with HIV of Reproductive Age.AIDS Behav. 2025 May 6:10.1007/s10461-025-04724-9. doi: 10.1007/s10461-025-04724-9. Online ahead of print. AIDS Behav. 2025. PMID: 40327266
-
Mental health, substance use, and risky sexual behaviors among women living with HIV.J Nurs Scholarsh. 2023 May;55(3):751-760. doi: 10.1111/jnu.12900. Epub 2023 Apr 20. J Nurs Scholarsh. 2023. PMID: 37132071 Free PMC article.
-
Interest in and Preference for Long-acting Injectable Antiretroviral Therapy in the Era of Approved Cabotegravir/Rilpivirine Among Reproductive-aged Women in the US South.Clin Infect Dis. 2025 Feb 5;80(1):164-167. doi: 10.1093/cid/ciae331. Clin Infect Dis. 2025. PMID: 38943370 Free PMC article.
-
Prevalence of genital and extragenital sexually transmitted infections among women of reproductive age with and without HIV in the Southern US: results from the study of treatment and reproductive outcomes.Front Med (Lausanne). 2025 Mar 26;12:1537427. doi: 10.3389/fmed.2025.1537427. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40206478 Free PMC article.
-
State-of-the-Science of human papillomavirus vaccination in women with human immunodeficiency Virus: Summary of a scientific workshop.Prev Med Rep. 2023 Jul 19;35:102331. doi: 10.1016/j.pmedr.2023.102331. eCollection 2023 Oct. Prev Med Rep. 2023. PMID: 37576844 Free PMC article. Review.
References
-
- HIV Surveillance Report, 2017; vol. 29. Centers for Disease Control and Prevention. 2018. [2019-04-14]. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveil... .
-
- Rana AI, Gillani FS, Flanigan TP, Nash BT, Beckwith CG. Follow-up care among HIV-infected pregnant women in Mississippi. J Womens Health (Larchmt) 2010 Oct;19(10):1863–7. doi: 10.1089/jwh.2009.1880. http://europepmc.org/abstract/MED/20831428 - DOI - PMC - PubMed
-
- Momplaisir Florence M, Storm Deborah S, Nkwihoreze Hervette, Jayeola Olakunle, Jemmott John B. Improving postpartum retention in care for women living with HIV in the United States. AIDS. 2018 Jan 14;32(2):133–142. doi: 10.1097/QAD.0000000000001707. http://europepmc.org/abstract/MED/29194122 - DOI - PMC - PubMed
-
- Firnhaber C, Smeaton LM, Grinsztejn B, Lalloo U, Faesen S, Samaneka W, Infante R, Rana A, Kumarasamy N, Hakim J, Campbell TB. Differences in antiretroviral safety and efficacy by sex in a multinational randomized clinical trial. HIV Clinical Trials. 2015 May 15;16(3):89–99. doi: 10.1179/1528433614z.0000000013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

