Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy
- PMID: 34932066
- PMCID: PMC8693209
- DOI: 10.1001/jamapediatrics.2021.5683
Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy
Abstract
Importance: BNT162b2 messenger RNA (mRNA) COVID-19 vaccination in the third trimester was found to be associated with a strong maternal humoral IgG response that crossed the placenta and approached maternal titers in the newborn.
Objective: To evaluate maternal and neonatal SARS-CoV-2 immunoglobulin G (IgG) antibody levels at birth after mRNA COVID-19 vaccination during the second trimester of pregnancy.
Design, setting, and participants: This prospective cohort study, conducted at a single medical center in Haifa, Israel, from May to July 2021, included women with a singleton pregnancy over 24 weeks of gestation at least 7 days after receipt of their second COVID-19 vaccine dose who were not known to be previously infected with COVID-19.
Exposures: BNT162b2 (Pfizer/BioNTech) vaccination.
Main outcomes and measures: The primary outcomes were SARS-CoV-2 IgG antibody titers measured in the parturient at admission and in the umbilical cord blood within 30 minutes after delivery. Secondary outcomes were the correlation between antibody titers, feto-maternal characteristics, maternal adverse effects after vaccination, and time interval from vaccination to delivery.
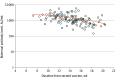
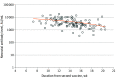
Results: Antibody levels were measured for 129 women (mean [SD] age, 31.9 [4.9] years) and 114 neonates, with 100% of the tests having positive results. The mean (SD) gestational age at administration of the second vaccine dose was 24.9 (3.3) weeks. Neonatal IgG titers were 2.6 times higher than maternal titers (median [range], 3315.7 [350.1-17 643.5] AU/mL vs 1185.2 [146.6-32 415.1] AU/mL). A positive correlation was demonstrated between maternal and neonatal antibodies (r = 0.92; 95% CI, 0.89-0.94). Multivariable analysis revealed that for each week that passed since receipt of the second vaccine dose, maternal and neonatal antibody levels changed by -10.9% (95% CI, -17.2% to -4.2%; P = .002) and -11.7% (95% CI, -19.0 to -3.8%; P = .005), respectively. For each 1-year increase in the mother's age, maternal and neonatal antibody levels changed by -3.1% (95% CI, -5.3% to -0.9%; P = .007) and -2.7% (95% CI, -5.2% to -0.1%; P = .04), respectively.
Conclusions and relevance: In this cohort study, receipt of the BNT162b2 mRNA COVID-19 vaccine during the second trimester of pregnancy was associated with maternal and neonatal humoral responses, as reflected in maternal and neonatal SARS-CoV-2 IgG antibody levels measured after delivery. These findings support COVID-19 vaccination of pregnant individuals during the second trimester to achieve maternal protection and newborn safety during the pandemic.
Conflict of interest statement
Figures
Comment in
-
Corona-Impfung schwangerer Frauen immunisiert auch das Ungeborene.MMW Fortschr Med. 2022 Feb;164(2):27. doi: 10.1007/s15006-022-0750-1. MMW Fortschr Med. 2022. PMID: 35088321 Free PMC article. Review. German. No abstract available.
References
-
- Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous