Effect of Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients With Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial
- PMID: 34932077
- PMCID: PMC8693274
- DOI: 10.1001/jama.2021.21316
Effect of Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy on Disease Control in Patients With Immune-Mediated Inflammatory Diseases: A Randomized Clinical Trial
Abstract
Importance: Proactive therapeutic drug monitoring (TDM), consisting of individualized treatment based on scheduled assessments of serum drug levels, has been proposed as an alternative to standard therapy to optimize efficacy and safety of infliximab and other biologic drugs. However, it remains unclear whether proactive TDM improves clinical outcomes during maintenance therapy.
Objective: To assess whether proactive TDM during maintenance therapy with infliximab improves treatment efficacy by preventing disease worsening compared with standard infliximab therapy without TDM.
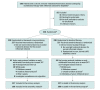
Design, setting, and participants: Randomized, parallel-group, open-label clinical trial including 458 adults with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn disease, or psoriasis undergoing maintenance therapy with infliximab in 20 Norwegian hospitals. Patients were recruited from June 7, 2017, to December 12, 2019. Final follow-up took place on December 14, 2020.
Interventions: Patients were randomized 1:1 to proactive TDM with dose and interval adjustments based on scheduled monitoring of serum drug levels and antidrug antibodies (TDM group; n = 228) or to standard infliximab therapy without drug and antibody level monitoring (standard therapy group; n = 230).
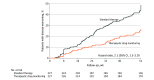
Main outcome and measures: The primary outcome was sustained disease control without disease worsening, defined by disease-specific composite scores or consensus about disease worsening between patient and physician leading to a major change in treatment (switching to another biologic drug, adding an immunosuppressive drug including glucocorticoids, or increasing the infliximab dose), during the 52-week study period.
Results: Among 458 randomized patients (mean age, 44.8 [SD, 14.3] years; 216 women [49.8%]), 454 received their randomly allocated intervention and were included in the full analysis set. The primary outcome of sustained disease control without disease worsening was observed in 167 patients (73.6%) in the TDM group and 127 patients (55.9%) in the standard therapy group. The estimated adjusted difference was 17.6% (95% CI, 9.0%-26.2%; P < .001) favoring TDM. Adverse events were reported in 137 patients (60%) and 142 patients (63%) in the TDM and standard therapy groups, respectively.
Conclusions and relevance: Among patients with immune-mediated inflammatory diseases undergoing maintenance therapy with infliximab, proactive TDM was more effective than treatment without TDM in sustaining disease control without disease worsening. Further research is needed to compare proactive TDM with reactive TDM, to assess the effects on long-term disease complications, and to evaluate the cost-effectiveness of this approach.
Trial registration: ClinicalTrials.gov Identifier: NCT03074656.
Conflict of interest statement
Figures

Comment in
-
Therapeutic Drug Monitoring for Immune-Mediated Inflammatory Diseases.JAMA. 2021 Dec 21;326(23):2370-2372. doi: 10.1001/jama.2021.21315. JAMA. 2021. PMID: 34932096 No abstract available.
-
Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy and Control of Immune-Mediated Inflammatory Diseases.JAMA. 2022 Apr 19;327(15):1505-1506. doi: 10.1001/jama.2022.2935. JAMA. 2022. PMID: 35438732 No abstract available.
-
Therapeutic Drug Monitoring vs Standard Therapy During Maintenance Infliximab Therapy and Control of Immune-Mediated Inflammatory Diseases.JAMA. 2022 Apr 19;327(15):1506. doi: 10.1001/jama.2022.2932. JAMA. 2022. PMID: 35438733 No abstract available.
References
-
- Movahedi M, Hepworth E, Mirza R, Cesta A, Larche M, Bombardier C. Discontinuation of biologic therapy due to lack/loss of response and adverse events is similar between TNFi and non-TNFi class: results from a real-world rheumatoid arthritis cohort. Semin Arthritis Rheum. 2020;50(5):915-922. doi:10.1016/j.semarthrit.2020.06.020 - DOI - PubMed

