Effect of Long-term Supplementation With Marine Omega-3 Fatty Acids vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial
- PMID: 34932079
- PMCID: PMC8693224
- DOI: 10.1001/jama.2021.21187
Effect of Long-term Supplementation With Marine Omega-3 Fatty Acids vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial
Abstract
Importance: Marine omega-3 fatty acid (omega-3) supplements have been used to treat depression but their ability to prevent depression in the general adult population is unknown.
Objective: To test effects of omega-3 supplementation on late-life depression risk and mood scores.
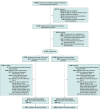
Design, setting, and participants: A total of 18 353 adults participated in the VITAL-DEP (Vitamin D and Omega-3 Trial-Depression Endpoint Prevention) ancillary study to VITAL, a randomized trial of cardiovascular disease and cancer prevention among 25 871 US adults. There were 16 657 at risk of incident depression (no previous depression) and 1696 at risk of recurrent depression (previous depression, but not for the past 2 years). Randomization occurred from November 2011 through March 2014; randomized treatment ended on December 31, 2017.
Interventions: Randomized 2 × 2 factorial assignment to vitamin D3 (2000 IU/d), marine omega-3 fatty acids (1 g/d of fish oil, including 465 mg of eicosapentaenoic acid and 375 mg of docosahexaenoic acid) or placebo; 9171 were randomized to omega-3 and 9182 were randomized to matching placebo.
Main outcomes and measures: Prespecified coprimary outcomes were risk of depression or clinically relevant depressive symptoms (total of incident + recurrent cases); mean difference in mood score (8-item Patient Health Questionnaire [PHQ-8] depression scale).
Results: Among 18 353 participants who were randomized (mean age, 67.5 [SD, 7.1] years; 49.2% women), 90.3% completed the trial (93.5% among those alive at the end of the trial); the median treatment duration was 5.3 years. The test for interaction between the omega-3 and the vitamin D agents was not significant (P for interaction = .14). Depression risk was significantly higher comparing omega-3 (651 events, 13.9 per 1000 person-years) with placebo (583 events, 12.3 per 1000 person-years; hazard ratio [HR], 1.13; 95% CI, 1.01-1.26; P = .03). No significant differences were observed comparing omega-3 with placebo groups in longitudinal mood scores: the mean difference in change in PHQ-8 score was 0.03 points (95% CI, -0.01 to 0.07; P = .19). Regarding serious and common adverse events, the respective prevalence values in omega-3 vs placebo groups were major cardiovascular events (2.7% vs 2.9%), all-cause mortality (3.3% vs 3.1%), suicide (0.02% vs 0.01%), gastrointestinal bleeding (2.6% vs 2.7%), easy bruising (24.8% vs 25.1%), and stomach upset or pain (35.2% vs 35.1%).
Conclusions and relevance: Among adults aged 50 years or older without clinically relevant depressive symptoms at baseline, treatment with omega-3 supplements compared with placebo yielded mixed results, with a small but statistically significant increase in risk of depression or clinically relevant depressive symptoms but no difference in mood scores, over a median follow-up of 5.3 years. These findings do not support the use of omega-3 supplements in adults to prevent depression.
Trial registration: ClinicalTrials.gov Identifiers: NCT01696435 and NCT01169259.
Conflict of interest statement
Figures


Comment in
-
Effect of Long-term Supplementation With Marine Omega-3 Fatty Acids vs Placebo on Risk of Depression.JAMA. 2022 Apr 5;327(13):1290-1291. doi: 10.1001/jama.2022.2030. JAMA. 2022. PMID: 35380588 No abstract available.
-
Effect of Long-term Supplementation With Marine Omega-3 Fatty Acids vs Placebo on Risk of Depression.JAMA. 2022 Apr 5;327(13):1291-1292. doi: 10.1001/jama.2022.2027. JAMA. 2022. PMID: 35380589 No abstract available.
References
-
- Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43(5):313-314. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 DK088762/DK/NIDDK NIH HHS/United States
- P30 MH090333/MH/NIMH NIH HHS/United States
- R01 AG036755/AG/NIA NIH HHS/United States
- R01 CA138962/CA/NCI NIH HHS/United States
- U01 CA138962/CA/NCI NIH HHS/United States
- R01 HL102122/HL/NHLBI NIH HHS/United States
- R01 HL101932/HL/NHLBI NIH HHS/United States
- R01 MH091448/MH/NIMH NIH HHS/United States
- R01 AT011729/AT/NCCIH NIH HHS/United States
- R01 AR059086/AR/NIAMS NIH HHS/United States
- R01 AR060574/AR/NIAMS NIH HHS/United States
- R01 DK088078/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

