A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine
- PMID: 34932102
- PMCID: PMC9016447
- DOI: 10.1093/infdis/jiab612
A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine
Abstract
Background: Protection against human respiratory syncytial virus (RSV) remains an unmet need potentially addressable by maternal immunization. This phase 1/2 study evaluated a bivalent prefusion F vaccine (RSVpreF) with antigens from RSV subgroups A and B.
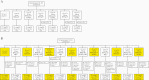
Methods: Adults 18-49 years old (N = 618) were randomized to receive placebo or 60, 120, or 240 µg RSVpreF with or without Al(OH)3. Safety and immunogenicity were evaluated.
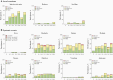
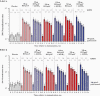
Results: RSVpreF recipients more frequently reported local reactions and systemic events than placebo recipients; these were mostly mild or moderate. No vaccine-related serious adverse events occurred through 12 months postvaccination. All RSVpreF formulations induced 1-month postvaccination virus-neutralizing titers higher than those associated with protection of high-risk infants by palivizumab, the only prophylactic currently available for RSV. Geometric mean fold rises (GMFRs) across RSVpreF doses/formulations were 10.6-16.9 for RSV A and 10.3-19.8 for RSV B at 1 month postvaccination, greater than those historically elicited by postfusion F vaccines. GMFRs were 3.9-5.2 and 3.7-5.1, respectively, at 12 months postvaccination.
Conclusions: RSVpreF formulations were safe, well tolerated, and induced robust neutralizing responses in adults. These findings support development of RSVpreF, which is being evaluated in a pivotal phase 3 study for maternal immunization.
Clinical trials registration: NCT03529773.
Keywords: F protein; immunogenicity; maternal vaccination; neutralizing responses; respiratory syncytial virus; safety; vaccine.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


Comment in
-
Are We Ready for Maternal Respiratory Syncytial Virus Vaccination?J Infect Dis. 2022 Jun 15;225(12):2053-2055. doi: 10.1093/infdis/jiab613. J Infect Dis. 2022. PMID: 34932123 No abstract available.
References
-
- Hall CB, Simoes EAF, Anderson LJ.. Clinical and epidemiologic features of respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin, Heidelberg: Springer, 2013:39–57.
-
- Glezen WP, Taber LH, Frank AL, Kasel JA.. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. - PubMed
-
- Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

