Effectiveness of mRNA Vaccines Against COVID-19 Hospitalization by Age and Chronic Medical Conditions Burden Among Immunocompetent US Adults, March-August 2021
- PMID: 34932114
- PMCID: PMC9113447
- DOI: 10.1093/infdis/jiab619
Effectiveness of mRNA Vaccines Against COVID-19 Hospitalization by Age and Chronic Medical Conditions Burden Among Immunocompetent US Adults, March-August 2021
Abstract
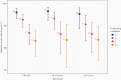
Vaccine effectiveness (VE) against COVID-19 hospitalization was evaluated among immunocompetent adults (≥18 years) during March-August 2021 using a case-control design. Among 1669 hospitalized COVID-19 cases (11% fully vaccinated) and 1950 RT-PCR-negative controls (54% fully vaccinated), VE was 96% (95% confidence interval [CI], 93%-98%) among patients with no chronic medical conditions and 83% (95% CI, 76%-88%) among patients with ≥ 3 categories of conditions. VE was similar between those aged 18-64 years versus ≥65 years (P > .05). VE against severe COVID-19 was very high among adults without chronic conditions and lessened with increasing comorbidity burden.
Keywords: COVID-19; chronic medical conditions; preexisting conditions; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures
References
-
- Centers for Disease Control and Prevention. COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 28 September 2021.
-
- Self WH, Tenforde MW, Rhoads JP, et al. . Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. - PMC - PubMed

