Streptococcus pneumoniae Serotypes Associated with Death, South Africa, 2012-2018
- PMID: 34932448
- PMCID: PMC8714227
- DOI: 10.3201/eid2801.210956
Streptococcus pneumoniae Serotypes Associated with Death, South Africa, 2012-2018
Abstract
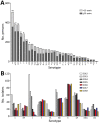
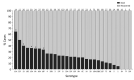
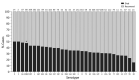
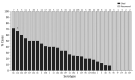
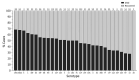
The Streptococcus pneumoniae polysaccharide capsule plays a role in disease severity. We assessed the association of serotype with case-fatality ratio (CFR) in invasive pneumococcal disease (IPD) and meningitis in South Africa, 2012-2018 (vaccine era), using multivariable logistic regression by manual backward elimination. The most common serotypes causing IPD were 8 and 19A. In patients <15 years of age, serotypes associated with increased CFR in IPD, compared with serotype 8 and controlling for confounding factors, were 11A, 13, 19F, 15A, and 6A. None of these serotypes were associated with increased CFR in meningitis. Among IPD patients >15 years of age, serotype 15B/C was associated with increased CFR. Among meningitis patients of all ages, serotype 1 was associated with increased CFR. PCV13 serotypes 1, 3, 6A, 19A, and 19F should be monitored, and serotypes 8, 12F, 15A, and 15B/C should be considered for inclusion in vaccines to reduce deaths caused by S. pneumoniae.
Keywords: South Africa; Streptococcus; Streptococcus pneumoniae; Switzerland; bacteria; case-fatality ratio; meningitis/encephalitis; mortality; polysaccharide capsule; serotype; streptococci.
Figures

References
-
- Clarke E, Bashorun AO, Okoye M, Umesi A, Badjie Hydara M, Adigweme I, et al. Safety and immunogenicity of a novel 10-valent pneumococcal conjugate vaccine candidate in adults, toddlers, and infants in The Gambia-Results of a phase 1/2 randomized, double-blinded, controlled trial. Vaccine. 2020;38:399–410. 10.1016/j.vaccine.2019.08.072 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

