FcγRIIIa receptor interacts with androgen receptor and PIP5K1α to promote growth and metastasis of prostate cancer
- PMID: 34932854
- PMCID: PMC9251882
- DOI: 10.1002/1878-0261.13166
FcγRIIIa receptor interacts with androgen receptor and PIP5K1α to promote growth and metastasis of prostate cancer
Abstract
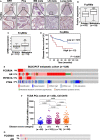
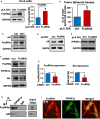
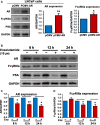
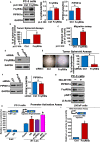
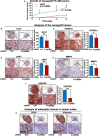
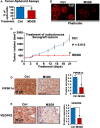
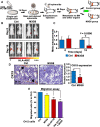
Low-affinity immunoglobulin gamma Fc region receptor III-A (FcγRIIIa) is a cell surface protein that belongs to a family of Fc receptors that facilitate the protective function of the immune system against pathogens. However, the role of FcγRIIIa in prostate cancer (PCa) progression remained unknown. In this study, we found that FcγRIIIa expression was present in PCa cells and its level was significantly higher in metastatic lesions than in primary tumors from the PCa cohort (P = 0.006). PCa patients with an elevated level of FcγRIIIa expression had poorer biochemical recurrence (BCR)-free survival compared with those with lower FcγRIIIa expression, suggesting that FcγRIIIa is of clinical importance in PCa. We demonstrated that overexpression of FcγRIIIa increased the proliferative ability of PCa cell line C4-2 cells, which was accompanied by the upregulation of androgen receptor (AR) and phosphatidylinositol-4-phosphate 5-kinase alpha (PIP5Kα), which are the key players in controlling PCa progression. Conversely, targeted inhibition of FcγRIIIa via siRNA-mediated knockdown or using its inhibitory antibody suppressed growth of xenograft PC-3 and PC-3M prostate tumors and reduced distant metastasis in xenograft mouse models. We further showed that elevated expression of AR enhanced FcγRIIIa expression, whereas inhibition of AR activity using enzalutamide led to a significant downregulation of FcγRIIIa protein expression. Similarly, inhibition of PIP5K1α decreased FcγRIIIa expression in PCa cells. FcγRIIIa physically interacted with PIP5K1α and AR via formation of protein-protein complexes, suggesting that FcγRIIIa is functionally associated with AR and PIP5K1α in PCa cells. Our study identified FcγRIIIa as an important factor in promoting PCa growth and invasion. Further, the elevated activation of FcγRIII and AR and PIP5K1α pathways may cooperatively promote PCa growth and invasion. Thus, FcγRIIIa may serve as a potential new target for improved treatment of metastatic and castration-resistant PCa.
Keywords: AR pathway and antibody-based therapy; FcγRIIIa receptor; PIP5K1α; prostate cancer metastasis; targeted therapy.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The functional interlink between AR and MMP9/VEGF signaling axis is mediated through PIP5K1α/pAKT in prostate cancer.Int J Cancer. 2020 Mar 15;146(6):1686-1699. doi: 10.1002/ijc.32607. Epub 2019 Aug 16. Int J Cancer. 2020. PMID: 31381135 Free PMC article.
-
Targeted suppression of AR-V7 using PIP5K1α inhibitor overcomes enzalutamide resistance in prostate cancer cells.Oncotarget. 2016 Sep 27;7(39):63065-63081. doi: 10.18632/oncotarget.11757. Oncotarget. 2016. PMID: 27588408 Free PMC article.
-
The interplay between AR, EGF receptor and MMP-9 signaling pathways in invasive prostate cancer.Mol Med. 2018 Jun 27;24(1):34. doi: 10.1186/s10020-018-0035-4. Mol Med. 2018. PMID: 30134822 Free PMC article.
-
AR function in promoting metastatic prostate cancer.Cancer Metastasis Rev. 2014 Sep;33(2-3):399-411. doi: 10.1007/s10555-013-9471-3. Cancer Metastasis Rev. 2014. PMID: 24425228 Free PMC article. Review.
-
Monoamine oxidase A (MAOA): A promising target for prostate cancer therapy.Cancer Lett. 2023 Jun 1;563:216188. doi: 10.1016/j.canlet.2023.216188. Epub 2023 Apr 17. Cancer Lett. 2023. PMID: 37076041 Review.
Cited by
-
Molecular landscape for risk prediction and personalized therapeutics of castration-resistant prostate cancer: at a glance.Front Endocrinol (Lausanne). 2024 Jun 3;15:1360430. doi: 10.3389/fendo.2024.1360430. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38887275 Free PMC article. Review.
-
Phosphoinositide kinases in cancer: from molecular mechanisms to therapeutic opportunities.Nat Rev Cancer. 2025 Jun;25(6):463-487. doi: 10.1038/s41568-025-00810-1. Epub 2025 Apr 3. Nat Rev Cancer. 2025. PMID: 40181165 Review.
-
JARID1D-dependent androgen receptor and JunD signaling activation of osteoclast differentiation inhibits prostate cancer bone metastasis through demethylating H3K4.Theranostics. 2025 Jan 1;15(4):1320-1337. doi: 10.7150/thno.104135. eCollection 2025. Theranostics. 2025. PMID: 39816691 Free PMC article.
-
Curcumin blunts epithelial-mesenchymal transition to alleviate invasion and metastasis of prostate cancer through the JARID1D demethylation.Cancer Cell Int. 2024 Sep 1;24(1):303. doi: 10.1186/s12935-024-03483-2. Cancer Cell Int. 2024. PMID: 39218854 Free PMC article.
References
-
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. - PubMed
-
- Baniyash M. TCR zeta‐chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol. 2004;4:675–87. - PubMed
-
- Baudino L, Nimmerjahn F, Azeredo da Silveira S, Martinez‐Soria E, Saito T, Carroll M, et al. Differential contribution of three activating IgG Fc receptors (FcgammaRI, FcgammaRIII, and FcgammaRIV) to IgG2a‐ and IgG2b‐induced autoimmune hemolytic anemia in mice. J Immunol. 2008;180:1948–53. - PubMed
-
- Abrahams VM, Cambridge G, Lydyard PM, Edwards JC. Induction of tumor necrosis factor alpha production by adhered human monocytes: a key role for Fcgamma receptor type IIIa in rheumatoid arthritis. Arthritis Rheum. 2000;43:608–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

