Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse
- PMID: 34932941
- PMCID: PMC8944915
- DOI: 10.1016/j.neuron.2021.11.031
Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse
Abstract
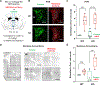
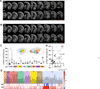
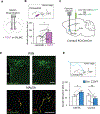
The neural basis of abnormal social behavior in autism spectrum disorders (ASDs) remains incompletely understood. Here we used two complementary but independent brain-wide mapping approaches, mouse resting-state fMRI and c-Fos-iDISCO+ imaging, to construct brain-wide activity and connectivity maps of the Cntnap2 knockout (KO) mouse model of ASD. At the macroscale level, we detected reduced functional coupling across social brain regions despite general patterns of hyperconnectivity across major brain structures. Oxytocin administration, which rescues social deficits in KO mice, strongly stimulated many brain areas and normalized connectivity patterns. Notably, chemogenetically triggered release of endogenous oxytocin strongly stimulated the nucleus accumbens (NAc), a forebrain nucleus implicated in social reward. Furthermore, NAc-targeted approaches to activate local oxytocin receptors sufficiently rescued their social deficits. Our findings establish circuit- and systems-level mechanisms of social deficits in Cntnap2 KO mice and reveal the NAc as a region that can be modulated by oxytocin to promote social interactions.
Keywords: autism; brain network; fMRI; functional connectivity; iDISCO; mouse model; nucleus accumbens; oxytocin; paraventricular nucleus; social behavior.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors report no competing interests.
Figures



References
-
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, and Hoehn M (2010). High field BOLD response to forepaw stimulation in the mouse. Neuroimage 51, 704–712. - PubMed
-
- Ahrens ET, and Dubowitz DJ (2001). Peripheral somatosensory fMRI in mouse at 11.7 T. NMR Biomed 14, 318–324. - PubMed
-
- Anderson DJ (2016). Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci 17, 692–704. - PubMed
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 67, 1–23. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

