Effect of SARS-CoV-2 seropositivity on antigen - specific cytokine and chemokine responses in latent tuberculosis
- PMID: 34933240
- PMCID: PMC8668379
- DOI: 10.1016/j.cyto.2021.155785
Effect of SARS-CoV-2 seropositivity on antigen - specific cytokine and chemokine responses in latent tuberculosis
Abstract
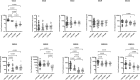
SARS-CoV-2 and latent Mycobacterium tuberculosis infection are both highly co-prevalent in many parts of the globe. Whether exposure to SARS-CoV-2 influences the antigen specific immune responses in latent tuberculosis has not been investigated. We examined the baseline, mycobacterial antigen and mitogen induced cytokine and chemokine responses in latent tuberculosis (LTBI) individuals with or without SARS-CoV-2 seropositivity, LTBI negative individuals with SARS-CoV-2 seropositivity and healthy control (both LTBI and SARS-CoV-2 negative) individuals. Our results demonstrated that LTBI individuals with SARS-CoV-2 seropositivity (LTBI+/IgG +) were associated with increased levels of unstimulated and TB-antigen stimulated IFNγ, IL-2, TNFα, IL-17, IL-1β, IL-6, IL-12, IL-4, CXCL1, CXCL9 and CXCL10 when compared to those without seropositivity (LTBI+/IgG-). In contrast, LTBI+/IgG+ individuals were associated with decreased levels of IL-5 and IL-10. No significant difference in the levels of cytokines/chemokines was observed upon mitogen stimulation between the groups. SARS-CoV-2 seropositivity was associated with enhanced unstimulated and TB-antigen stimulated but not mitogen stimulated production of cytokines and chemokines in LTBI+ compared to LTBI negative individuals. Finally, most of these significant differences were not observed when LTBI negative individuals with SARS-CoV-2 seropositivity and controls were examined. Our data clearly demonstrate that both baseline and TB - antigen induced cytokine responses are augmented in the presence of SARS-CoV-2 seropositivity, suggesting an augmenting effect of prior SARS-CoV-2 infection on the immune responses of LTBI individuals.
Keywords: Antigen specific responses; Chemokines; Cytokines; LTBI; SARS-CoV-2 seropositivity.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- W.H. Organization, Global tuberculosis report 2020, 2020. World Health Organization. Global tuberculosis report 2020. Geneva: WHO, 2020: 1–232. (Accessed February 24, 2021).
-
- W.H. Organization, Coronavirus disease (COVID-19) pandemic, (2021).
-
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. C China Medical Treatment Expert Group for, Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. - PMC - PubMed
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

