Towards a population-based threshold of protection for COVID-19 vaccines
- PMID: 34933765
- PMCID: PMC8673730
- DOI: 10.1016/j.vaccine.2021.12.006
Towards a population-based threshold of protection for COVID-19 vaccines
Abstract
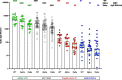
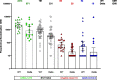
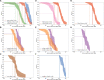
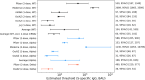
Correlates of protection for COVID-19 vaccines are urgently needed to license additional vaccines. We measured immune responses to four COVID-19 vaccines of proven efficacy using a single serological platform. IgG anti-Spike antibodies were highly correlated with ID50 neutralization in a validated pseudoviral assay and correlated significantly with efficacies for protection against infection with wild-type, alpha and delta variant SARS-CoV-2 virus. The protective threshold for each vaccine was calculated for IgG anti-Spike antibody. The mean protective threshold for all vaccine studies for WT virus was 154 BAU/ml (95 %CI 42-559), and for studies with antibody distributions that enabled precise estimation of thresholds (i.e. leaving out 2-dose mRNA regimens) was 60 BAU/ml (95 %CI 35-102). We propose that the proportion of individuals with responses above the appropriate protective threshold together with the geometric mean concentration can be used in comparative non-inferiority studies with licensed vaccines to ensure that new vaccines will be efficacious.
Keywords: COVID vaccines; COVID-19; Correlates of protection; SARS-CoV-2.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Dr. Plotkin consults for Janssen and Moderna; Dr. Siber reports personal fees and other from Clover Biopharmaceuticals, personal fees from AdVaccine, other from Vaxxinity personal fees from CanSino, personal fees from CureVac, personal fees from Valneva, personal fees from Vaxart, personal fees and other from Affinivax, outside the submitted work; Dr. Ambrosino reports personal fees from Vaxxinity, personal fees and other from Clover Biopharmaceuticals, outside the submitted work. Dr. Montefiori’s laboratory receives funding from Moderna for clinical sample testing].
Figures

References
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. - PubMed
-
- Chen R.T., Markowitz L.E., Albrecht P., Stewart J.A., Mofenson L.M., Preblud S.R., et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–1042. - PubMed
-
- Woudenberg T., van Binnendijk R., Veldhuijzen I., Woonink F., Ruijs H., van der Klis F., et al. Additional Evidence on Serological Correlates of Protection against Measles: An Observational Cohort Study among Once Vaccinated Children Exposed to Measles. Vaccines (Basel) 2019;7(4):158. doi: 10.3390/vaccines7040158. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

