C-reactive protein (CRP) as a biomarker of pulmonary exacerbation presentation and treatment response
- PMID: 34933824
- PMCID: PMC9206040
- DOI: 10.1016/j.jcf.2021.12.003
C-reactive protein (CRP) as a biomarker of pulmonary exacerbation presentation and treatment response
Abstract
Background: C-reactive protein (CRP) has been proposed as a biomarker for pulmonary exacerbation (PEx) diagnosis and treatment response. CRP >75mg/L has been associated with increased risk of PEx treatment failure. We have analyzed CRP measures as biomarkers for clinical response during the STOP2 PEx study (NCT02781610).
Methods: CRP measures were collected at antimicrobial treatment start (V1), seven to 10 days later (V2), and two weeks after treatment end (V3). V1 log10CRP concentrations and log10CRP change from V1 to V3 correlations with clinical responses (changes in lung function and symptom score) were assessed by least squares regression. Odds of intravenous (IV) antimicrobial retreatment within 30 days and future PEx hazard associated with V1 and V3 CRP concentrations and V1 CRP >75 mg/L were studied by adjusted logistic regression and proportional hazards modeling, respectively.
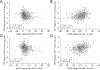
Results: In all, 951 of 982 STOP2 subjects (92.7%) had CRP measures at V1. V1 log10CRP varied significantly by V1 lung function subgroup, symptom score quartile, and sex, but not by age subgroup. V1 log10CRP correlated moderately with log10CRP change at V3 (r2=0.255) but less so with lung function (r2=0.016) or symptom (r2=0.031) changes at V3. Higher V1 CRP was associated with greater response. CRP changes from V1 to V3 only weakly correlated with lung function (r2=0.061) and symptom (r2=0.066) changes. However, V3 log10CRP was associated with increased odds of retreatment (P = .0081) and future PEx hazard (P = .0114).
Discussion: Despite consistent trends, log10CRP change was highly variable with only limited utility as a biomarker of PEx treatment response.
Keywords: C-reactive protein; Clinical response; Cystic fibrosis; Pulmonary exacerbation.
Copyright © 2021 European Cystic Fibrosis Society. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors claim no financial conflicts of interest related to this work.
Figures

References
-
- Cystic Fibrosis Foundation. Treatment of pulmonary exacerbation of cystic fibrosis. Clinical practice guidelines for cystic fibrosis; 1997.
-
- Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011. Sep;46(9):870–81. - PubMed
-
- Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, Bell NJ, Rainer M, Mt-Isa S, Voase N, Dewar MH, Saunders C, Gibson JS, Parra-Leiton J, Larsen MD, Jeswiet S, Soussi S, Bakar Y, Meister MG, Tyler P, Doherty A, Hansell DM, Ashby D, Hyde SC, Gill DR, Greening AP, Porteous DJ, Innes JA, Boyd AC, Griesenbach U, Cunningham S, Alton EW. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013. Jun;68(6):532–9. - PubMed
-
- Shoki AH, Mayer-Hamblett N, Wilcox PG, Sin DD, Quon BS. Systematic review of blood biomarkers in cystic fibrosis pulmonary exacerbations. Chest. 2013. Nov;144(5):1659–1670. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

