On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles
- PMID: 34933999
- PMCID: PMC8719871
- DOI: 10.1073/pnas.2109256118
On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles
Abstract
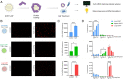
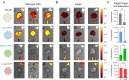
Lipid nanoparticles (LNPs) are a clinically mature technology for the delivery of genetic medicines but have limited therapeutic applications due to liver accumulation. Recently, our laboratory developed selective organ targeting (SORT) nanoparticles that expand the therapeutic applications of genetic medicines by enabling delivery of messenger RNA (mRNA) and gene editing systems to non-liver tissues. SORT nanoparticles include a supplemental SORT molecule whose chemical structure determines the LNP's tissue-specific activity. To understand how SORT nanoparticles surpass the delivery barrier of liver hepatocyte accumulation, we studied the mechanistic factors which define their organ-targeting properties. We discovered that the chemical nature of the added SORT molecule controlled biodistribution, global/apparent pKa, and serum protein interactions of SORT nanoparticles. Additionally, we provide evidence for an endogenous targeting mechanism whereby organ targeting occurs via 1) desorption of poly(ethylene glycol) lipids from the LNP surface, 2) binding of distinct proteins to the nanoparticle surface because of recognition of exposed SORT molecules, and 3) subsequent interactions between surface-bound proteins and cognate receptors highly expressed in specific tissues. These findings establish a crucial link between the molecular composition of SORT nanoparticles and their unique and precise organ-targeting properties and suggest that the recruitment of specific proteins to a nanoparticle's surface can enable drug delivery beyond the liver.
Keywords: endogenous targeting; gene editing; lipid nanoparticles; mRNA delivery.
Figures


References
-
- Miller J. B., Siegwart D. J., Design of synthetic materials for intracellular delivery of RNAs: From siRNA-mediated gene silencing to CRISPR/Cas gene editing. Nano Res. 11, 5310–5337 (2018).
-
- Sahin U., Karikó K., Türeci Ö., mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014). - PubMed
-
- Hajj K. A., Whitehead K. A., Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

