Members of the KCTD family are major regulators of cAMP signaling
- PMID: 34934014
- PMCID: PMC8740737
- DOI: 10.1073/pnas.2119237119
Members of the KCTD family are major regulators of cAMP signaling
Abstract
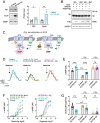
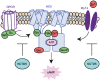
Cyclic adenosine monophosphate (cAMP) is a pivotal second messenger with an essential role in neuronal function. cAMP synthesis by adenylyl cyclases (AC) is controlled by G protein-coupled receptor (GPCR) signaling systems. However, the network of molecular players involved in the process is incompletely defined. Here, we used CRISPR/Cas9-based screening to identify that members of the potassium channel tetradimerization domain (KCTD) family are major regulators of cAMP signaling. Focusing on striatal neurons, we show that the dominant isoform KCTD5 exerts its effects through an unusual mechanism that modulates the influx of Zn2+ via the Zip14 transporter to exert unique allosteric effects on AC. We further show that KCTD5 controls the amplitude and sensitivity of stimulatory GPCR inputs to cAMP production by Gβγ-mediated AC regulation. Finally, we report that KCTD5 haploinsufficiency in mice leads to motor deficits that can be reversed by chelating Zn2+ Together, our findings uncover KCTD proteins as major regulators of neuronal cAMP signaling via diverse mechanisms.
Keywords: GPCR; cAMP; neuron; striatum; zinc.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Beavo J. A., Brunton L. L., Cyclic nucleotide research – Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718 (2002). - PubMed
-
- Post S. R., Hammond H. K., Insel P. A., Beta-adrenergic receptors and receptor signaling in heart failure. Annu. Rev. Pharmacol. Toxicol. 39, 343–360 (1999). - PubMed
-
- Kandel E. R., The molecular biology of memory storage: A dialogue between genes and synapses. Science 294, 1030–1038 (2001). - PubMed
-
- Davis R. L., Cherry J., Dauwalder B., Han P. L., Skoulakis E., The cyclic AMP system and Drosophila learning. Mol. Cell. Biochem. 149-150, 271–278 (1995). - PubMed
-
- Klein C., Sunahara R. K., Hudson T. Y., Heyduk T., Howlett A. C., Zinc inhibition of cAMP signaling. J. Biol. Chem. 277, 11859–11865 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

