Closantel is an allosteric inhibitor of human Taspase1
- PMID: 34934933
- PMCID: PMC8661544
- DOI: 10.1016/j.isci.2021.103524
Closantel is an allosteric inhibitor of human Taspase1
Abstract
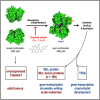
Dimerization of Taspase1 activates an intrinsic serine protease function that leads to the catalytic Thr234 residue, which allows to catalyze the consensus sequence Q-3X-2D-1⋅G1X2D3D4, present in Trithorax family members and TFIIA. Noteworthy, Taspase1 performs only a single hydrolytic step on substrate proteins, which makes it impossible to screen for inhibitors in a classical screening approach. Here, we report the development of an HTRF reporter assay that allowed the identification of an inhibitor, Closantel sodium, that inhibits Taspase1 in a noncovalent fashion (IC50 = 1.6 μM). The novel inhibitor interferes with the dimerization step and/or the intrinsic serine protease function of the proenzyme. Of interest, Taspase1 is required to activate the oncogenic functions of the leukemogenic AF4-MLL fusion protein and was shown in several studies to be overexpressed in many solid tumors. Therefore, the inhibitor may be useful for further validation of Taspase1 as a target for cancer therapy.
Keywords: Biochemistry; Biophysical chemistry; Structural biology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Bursen A., Moritz S., Gaussmann A., Moritz S., Dingermann T., Marschalek R. Interaction of AF4 wild-type and AF4⋅MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene. 2004;23:6237–6249. - PubMed
LinkOut - more resources
Full Text Sources

