Nonhormonal therapy for endometriosis: a randomized, placebo-controlled, pilot study of cabergoline versus norethindrone acetate
- PMID: 34934987
- PMCID: PMC8655411
- DOI: 10.1016/j.xfre.2021.07.003
Nonhormonal therapy for endometriosis: a randomized, placebo-controlled, pilot study of cabergoline versus norethindrone acetate
Abstract
Objective: To estimate the efficacy and safety of a novel nonhormonal therapeutic agent, cabergoline, compared with that of the standard clinical therapy, norethindrone acetate (NETA), for the treatment of endometriosis-associated pain in young women with endometriosis.
Design: Randomized, double-blind, placebo-controlled pilot study.
Setting: Tertiary care center.
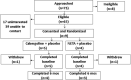
Patients: Women (n = 9) with surgically confirmed endometriosis.
Interventions: A random, double-blind assignment to either NETA (5 mg/day) + placebo twice weekly or cabergoline (0.5 mg) twice weekly + placebo daily for 6 months.
Main outcome measures: We collected the measures of pelvic pain and laboratory parameters every 3 months.
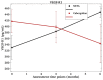
Results: We observed a decrease in pain scores and increase in pain relief in women randomized to receive cabergoline, who appeared to show similar or more improvements than women treated with NETA. The serum measures of vascular endothelial growth factor receptor 1 declined over 6 months in those who received cabergoline. Cabergoline was well tolerated, and no serious adverse events occurred.
Conclusions: Safe, effective adjunct treatments are lacking for patients with endometriosis who do not respond to standard care. Because the growth of endometriosis requires angiogenesis, blood vessel growth is an attractive therapeutic target. This pilot study suggests that cabergoline, a vascular endothelial growth factor pathway inhibitor, is an effective therapeutic option for women with chronic pain due to endometriosis. Building upon this investigation, we will conduct larger, randomized trials of cabergoline, advancing research on the best treatments for endometriosis-particularly disease resistant to hormonal therapies.
Clinical trial registration number: clinicaltrials.gov; registration number NCT02542410.
Keywords: Angiogenesis; cabergoline; endometriosis; nonhormonal therapy; norethindrone acetate.
© 2021 The Authors.
Figures

References
-
- Zondervan K.T., Becker C.M., Missmer S.A. Endometriosis. N Engl J Med. 2020;382:1244–1256. - PubMed
-
- Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397:839–852. - PubMed
-
- ACOG Committee Opinion. Number 310, April 2005. Endometriosis in adolescents. Obstet Gynecol. 2005;105:921–927. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

