Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England
- PMID: 34935001
- PMCID: PMC8676418
- DOI: 10.1016/S2666-7568(21)00282-8
Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England
Abstract
Background: Long-term care facilities (LTCFs) have reported high SARS-CoV-2 infection rates and related mortality, but the proportion of infected people among those who have survived, and duration of the antibody response to natural infection, is unknown. We determined the prevalence and stability of nucleocapsid antibodies (the standard assay for detection of previous infection) in staff and residents in LTCFs in England.
Methods: This was a prospective cohort study of residents 65 years or older and of staff 65 years or younger in 201 LTCFs in England between March 1, 2020, and May 7, 2021. Participants were linked to a unique pseudo-identifier based on their UK National Health Service identification number. Serial blood samples were tested for IgG antibodies against SARS-CoV-2 nucleocapsid protein using the Abbott ARCHITECT i-system (Abbott, Maidenhead, UK) immunoassay. Primary endpoints were prevalence and cumulative incidence of antibody positivity, which were weighted to the LTCF population. Incidence rate of loss of antibodies (seroreversion) was estimated from Kaplan-Meier curves.
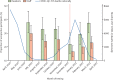
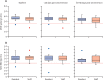
Findings: 9488 samples were included, 8636 (91·0%) of which could be individually linked to 1434 residents and 3288 staff members. The cumulative incidence of nucleocapsid seropositivity was 34·6% (29·6-40·0) in residents and 26·1% (23·0-29·5) in staff over 11 months. 239 (38·6%) residents and 503 women (81·3%) were included in the antibody-waning analysis, and median follow-up was 149 days (IQR 107-169). The incidence rate of seroreversion was 2·1 per 1000 person-days at risk, and median time to reversion was 242·5 days.
Interpretation: At least a quarter of staff and a third of surviving residents were infected with SAR-CoV-2 during the first two waves of the pandemic in England. Nucleocapsid-specific antibodies often become undetectable within the first year following infection, which is likely to lead to marked underestimation of the true proportion of people with previous infection. Given that natural infection might act to boost vaccine responses, better assays to identify natural infection should be developed.
Funding: UK Government Department of Health and Social Care.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
LS and TP report grants from the Department of Health and Social Care during the conduct of the study and LS is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AI-S is employed by the Department of Health and Social Care who funded the study. AH reports funding from the Covid Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Figures

Comment in
-
Estimating SARS-CoV-2 seroprevalence in long-term care: a window of opportunity.Lancet Healthy Longev. 2022 Jan;3(1):e2-e3. doi: 10.1016/S2666-7568(21)00304-4. Epub 2021 Dec 16. Lancet Healthy Longev. 2022. PMID: 34935000 Free PMC article. No abstract available.
References
-
- Bell D, Comas-Herrera A, Henderson D, et al. COVID-19 mortality and long-term care: a UK comparison. Aug 29, 2020. https://ltccovid.org/wp-content/uploads/2020/08/COVID-19-mortality-in-lo...
-
- Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

