Degenerative changes of the aortic valve during left ventricular assist device support
- PMID: 34935306
- PMCID: PMC8788006
- DOI: 10.1002/ehf2.13767
Degenerative changes of the aortic valve during left ventricular assist device support
Abstract
Aims: Donor heart shortage leads to increasing use of left ventricular assist device (LVAD) as bridge-to-transplant or destination therapy. Prolonged LVAD support is associated with aortic valve insufficiency, representing a relevant clinical problem in LVAD patients. Nevertheless, the impact of LVAD support on inflammation, remodelling, and chondro-osteogenic differentiation of the aortic valve is still not clearly understood. The aim of the study is to evaluate the impact of LVAD support on structural and molecular alterations of the aortic valve.
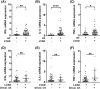
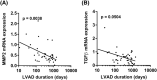
Methods and results: During heart transplantation, aortic valves of 63 heart failure patients without (n = 22) and with LVAD support (n = 41) were collected and used for analysis. Data on clinical course as well as echocardiographic data were analysed. Calcification and markers of remodelling, chondro-osteogenic differentiation, and inflammation were evaluated by computed tomography, by mRNA analysis and by histology and immunohistochemistry. Expression of inflammation markers of the LVAD group was analysed with regard to levels of C-reactive protein and driveline infections. Calcium accumulation and mRNA expression of determined markers were correlated with duration of LVAD support. Data were also analysed relating to aortic valve opening and aortic valve insufficiency. There was no difference in the frequency of cardiovascular risk factors or comorbidities between the patient groups. Expression of matrix metalloproteinase-9 (P = 0.007), alpha-smooth muscle actin (P = 0.045), and osteopontin (P = 0.003) were up-regulated in aortic valves of LVAD patients. Histological appearance of the aortic valve was similar in patients with or without LVAD, and computed tomography-based analysis not yet revealed significant difference in tissue calcification. Expression of interferon gamma (P = 0.004), interleukin-1 beta (P < 0.0001), and tumour necrosis factor alpha (P = 0.04) was up-regulated in aortic valves of LVAD patients without concomitant inflammatory cell infiltration and independent from unspecific inflammation. Expression of matrix metalloproteinase-2 (P = 0.038) and transforming growth factor beta (P = 0.0504) correlated negatively with duration of LVAD support. Presence of aortic valve insufficiency led to a significantly higher expression of interferon gamma (P = 0.007) in LVAD patients. There was no alteration in the determined markers in relation to aortic valve opening in LVAD patients.
Conclusions: Left ventricular assist device support leads to signs of early aortic valve degeneration independent of support duration. Thus, the aortic valve of patients with LVAD support should be closely monitored, particularly in patients receiving destination therapy as well as in the prospect of using aortic valves of LVAD patients as homografts in case of bridge-to-transplant therapy.
Keywords: Aortic valve; Degeneration; Inflammation; Left ventricular assist device; Remodelling.
© 2021 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
M.B., L.M., N.N., J.I.S., U.B., Y.S., N.K., P.H., R.W., P.K., and H.A. have no conflict of interest to declare. P.A. receives speaker honoraria from Medtronic, Abbott, Edwards, Ascyrus Medical, and Abiomed. A.L. and P.A. have received research grants from Abbott and Edwards outside the submitted work.
Figures



References
-
- Hanff TC, Birati EY. Left ventricular assist device as destination therapy: a state of the science and art of long‐term mechanical circulatory support. Curr Heart Fail Rep 2019; 16: 168–179. - PubMed
-
- Patel N, Kalra R, Doshi R, Bajaj NS, Arora G, Arora P. Trends and cost of heart transplantation and left ventricular assist devices: impact of proposed federal cuts. JACC Heart Fail 2018; 6: 424–432. - PubMed
-
- Beckmann A, Meyer R, Lewandowski J, Markewitz A, Gummert J. German heart surgery report 2019: the annual updated registry of the German society for thoracic and cardiovascular surgery. Thorac Cardiovasc Surg 2020; 68: 263–276. - PubMed
-
- Noly PE, Pagani FD, Noiseux N, Stulak JM, Khalpey Z, Carrier M, Maltais S. Continuous‐flow left ventricular assist devices and valvular heart disease: a comprehensive review. Can J Cardiol 2020; 36: 244–260. - PubMed
-
- Misumi Y, Miyagawa S, Yoshioka D, Kainuma S, Kawamura T, Kawamura A, Maruyama Y, Ueno T, Toda K, Asanoi H, Sawa Y. Prediction of aortic valve regurgitation after continuous‐flow left ventricular assist device implantation using artificial intelligence trained on acoustic spectra. J Artif Organs 2021; 24: 164–172. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

