SARS-CoV-2 Spike Protein Destabilizes Microvascular Homeostasis
- PMID: 34935423
- PMCID: PMC8693925
- DOI: 10.1128/Spectrum.00735-21
SARS-CoV-2 Spike Protein Destabilizes Microvascular Homeostasis
Abstract
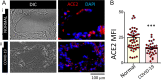
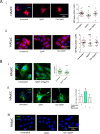
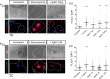
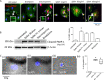
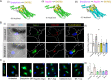
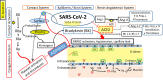
SARS-CoV-2 infection can cause compromised respiratory function and thrombotic events. SARS-CoV-2 binds to and mediates downregulation of angiotensin converting enzyme 2 (ACE2) on cells that it infects. Theoretically, diminished enzymatic activity of ACE2 may result in increased concentrations of pro-inflammatory molecules, angiotensin II, and Bradykinin, contributing to SARS-CoV-2 pathology. Using immunofluorescence microscopy of lung tissues from uninfected, and SARS-CoV-2 infected individuals, we find evidence that ACE2 is highly expressed in human pulmonary alveolar epithelial cells and significantly reduced along the alveolar lining of SARS-CoV-2 infected lungs. Ex vivo analyses of primary human cells, indicated that ACE2 is readily detected in pulmonary alveolar epithelial and aortic endothelial cells. Exposure of these cells to spike protein of SARS-CoV-2 was sufficient to reduce ACE2 expression. Moreover, exposure of endothelial cells to spike protein-induced dysfunction, caspase activation, and apoptosis. Exposure of endothelial cells to bradykinin caused calcium signaling and endothelial dysfunction (increased expression of von Willibrand Factor and decreased expression of Krüppel-like Factor 2) but did not adversely affect viability in primary human aortic endothelial cells. Computer-assisted analyses of molecules with potential to bind bradykinin receptor B2 (BKRB2), suggested a potential role for aspirin as a BK antagonist. When tested in our in vitro model, we found evidence that aspirin can blunt cell signaling and endothelial dysfunction caused by bradykinin in these cells. Interference with interactions of spike protein or bradykinin with endothelial cells may serve as an important strategy to stabilize microvascular homeostasis in COVID-19 disease. IMPORTANCE SARS-CoV-2 causes complex effects on microvascular homeostasis that potentially contribute to organ dysfunction and coagulopathies. SARS-CoV-2 binds to, and causes downregulation of angiotensin converting enzyme 2 (ACE2) on cells that it infects. It is thought that reduced ACE2 enzymatic activity can contribute to inflammation and pathology in the lung. Our studies add to this understanding by providing evidence that spike protein alone can mediate adverse effects on vascular cells. Understanding these mechanisms of pathogenesis may provide rationale for interventions that could limit microvascular events associated with SARS-CoV-2 infection.
Keywords: COVID-19; endothelial cells; spike protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells.Vascul Pharmacol. 2021 Apr;137:106823. doi: 10.1016/j.vph.2020.106823. Epub 2020 Nov 21. Vascul Pharmacol. 2021. PMID: 33232769 Free PMC article.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
-
SARS-CoV-2 Entry Receptor ACE2 Is Expressed on Very Small CD45- Precursors of Hematopoietic and Endothelial Cells and in Response to Virus Spike Protein Activates the Nlrp3 Inflammasome.Stem Cell Rev Rep. 2021 Feb;17(1):266-277. doi: 10.1007/s12015-020-10010-z. Stem Cell Rev Rep. 2021. PMID: 32691370 Free PMC article.
-
SARS-Cov-2 Replication in a Blood-Brain Barrier Model Established with Human Brain Microvascular Endothelial Cells Induces Permeability and Disables ACE2-Dependent Regulation of Bradykinin B1 Receptor.Int J Mol Sci. 2025 Jun 10;26(12):5540. doi: 10.3390/ijms26125540. Int J Mol Sci. 2025. PMID: 40565006 Free PMC article.
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
Cited by
-
Three-Day Icatibant on Top of Standard Care in Patients With Coronavirus Disease 2019 Pneumonia: A Randomized, Open-Label, Phase 2, Proof-of-Concept Trial.Clin Infect Dis. 2023 May 24;76(10):1784-1792. doi: 10.1093/cid/ciac984. Clin Infect Dis. 2023. PMID: 36610464 Free PMC article. Clinical Trial.
-
More than a key-the pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury.Sports Med Health Sci. 2023 Mar 30;6(3):209-20. doi: 10.1016/j.smhs.2023.03.004. Online ahead of print. Sports Med Health Sci. 2023. PMID: 37361919 Free PMC article. Review.
-
Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis.Trends Mol Med. 2022 Jul;28(7):542-554. doi: 10.1016/j.molmed.2022.04.007. Epub 2022 Apr 21. Trends Mol Med. 2022. PMID: 35537987 Free PMC article. Review.
-
A snake venom-analog peptide that inhibits SARS-CoV-2 and papain-like protease displays antithrombotic activity in mice arterial thrombosis model, without interfering with bleeding time.Thromb J. 2023 Jan 2;21(1):1. doi: 10.1186/s12959-022-00436-5. Thromb J. 2023. PMID: 36593467 Free PMC article.
-
Vascular mechanisms of post-COVID-19 conditions: Rho-kinase is a novel target for therapy.Eur Heart J Cardiovasc Pharmacother. 2023 Jun 2;9(4):371-386. doi: 10.1093/ehjcvp/pvad025. Eur Heart J Cardiovasc Pharmacother. 2023. PMID: 37019821 Free PMC article.
References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. 2008. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 372:127–135. doi:10.1016/j.virol.2007.09.045. - DOI - PMC - PubMed
-
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, Jordan TX, Oishi K, Panis M, Sachs D, Wang TT, Schwartz RE, Lim JK, Albrecht RA, tenOever BR. 2020. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181:1036–1045 e1039. doi:10.1016/j.cell.2020.04.026. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

