Catch Me if You Can: the Crosstalk of Zika Virus and the Restriction Factor Tetherin
- PMID: 34935441
- PMCID: PMC8865524
- DOI: 10.1128/jvi.02117-21
Catch Me if You Can: the Crosstalk of Zika Virus and the Restriction Factor Tetherin
Abstract
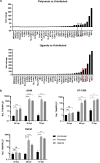
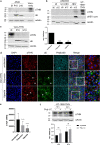
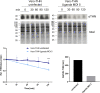
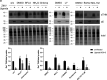
Zika virus (ZIKV) is a flavivirus that is mainly transmitted by Aedes mosquitos and normally causes mild symptoms. During the outbreak in the Americas in 2015, it was associated with more severe implications, like microcephaly in newborns and the Guillain-Barré syndrome. The lack of specific vaccines and cures strengthens the need for a deeper understanding of the virus life cycle and virus-host interactions. The restriction factor tetherin (THN) is an interferon-inducible cellular protein with broad antiviral properties. It is known to inhibit the release of various enveloped viruses by tethering them to each other and the cell membrane, thereby preventing their further spread. On the other hand, different viruses have developed various escape strategies against THN. Analysis of the cross-talk between ZIKV and THN revealed that, despite a strong induction of THN mRNA expression in ZIKV-infected cells, this is not reflected by an elevated protein level of THN. Contrariwise, the THN protein level is decreased due to a reduced half-life. The increased degradation of THN in ZIKV infected cells involves the endo-lysosomal system but does not depend on the early steps of autophagy. Enrichment of THN by depletion of the ESCRT-0 protein HRS diminishes ZIKV release and spread, which points out the capacity of THN to restrict ZIKV and explains the enhanced THN degradation in infected cells as an effective viral escape strategy. IMPORTANCE Although tetherin expression is strongly induced by ZIKV infection there is a reduction in the amount of tetherin protein. This is due to enhanced lysosomal degradation. However, if the tetherin level is rescued then the release of ZIKV is impaired. This shows that tetherin is a restriction factor for ZIKV, and the induction of an efficient degradation represents a viral escape strategy. To our knowledge, this is the first study that describes and characterizes tetherin as a restriction factor for the ZIKV life cycle.
Keywords: BST-2; HRS; ZIKV; Zika virus; flavivirus; tetherin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation.PLoS Pathog. 2011 Feb 3;7(2):e1001265. doi: 10.1371/journal.ppat.1001265. PLoS Pathog. 2011. PMID: 21304933 Free PMC article.
-
Roles played by capsid-dependent induction of membrane curvature and Gag-ESCRT interactions in tetherin recruitment to HIV-1 assembly sites.J Virol. 2013 Apr;87(8):4650-64. doi: 10.1128/JVI.03526-12. Epub 2013 Feb 13. J Virol. 2013. PMID: 23408603 Free PMC article.
-
Early Vertebrate Evolution of the Host Restriction Factor Tetherin.J Virol. 2015 Dec;89(23):12154-65. doi: 10.1128/JVI.02149-15. Epub 2015 Sep 23. J Virol. 2015. PMID: 26401043 Free PMC article.
-
Antiviral inhibition of enveloped virus release by tetherin/BST-2: action and counteraction.Viruses. 2011 May;3(5):520-40. doi: 10.3390/v3050520. Epub 2011 May 6. Viruses. 2011. PMID: 21994744 Free PMC article. Review.
-
Emerging role of the host restriction factor tetherin in viral immune sensing.J Mol Biol. 2013 Dec 13;425(24):4956-64. doi: 10.1016/j.jmb.2013.09.029. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075872 Review.
Cited by
-
Co-expression analysis suggests lncRNA-mRNA interactions enhance antiviral immune response during acute Chikungunya fever in whole blood of pediatric patients.PLoS One. 2023 Nov 3;18(11):e0294035. doi: 10.1371/journal.pone.0294035. eCollection 2023. PLoS One. 2023. PMID: 37922302 Free PMC article.
-
CD317 functions as a key antiviral factor in human herpesvirus 6 (HHV-6) infection.J Virol. 2025 Jul 22;99(7):e0084125. doi: 10.1128/jvi.00841-25. Epub 2025 Jun 24. J Virol. 2025. PMID: 40552817 Free PMC article.
References
-
- World Health Organization. Zika virus. https://www.who.int/news-room/fact-sheets/detail/zika-virus. Accessed 29 September, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

