Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination
- PMID: 34935921
- PMCID: PMC8696570
- DOI: 10.1001/jamanetworkopen.2021.40364
Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination
Abstract
Importance: Little is known about the factors associated with COVID-19 vaccine adverse effects in a real-world population.
Objective: To evaluate factors potentially associated with participant-reported adverse effects after COVID-19 vaccination.
Design, setting, and participants: The COVID-19 Citizen Science Study, an online cohort study, includes adults aged 18 years and older with a smartphone or internet access. Participants complete daily, weekly, and monthly surveys on health and COVID-19-related events. This analysis includes participants who provided consent between March 26, 2020, and May 19, 2021, and received at least 1 COVID-19 vaccine dose.
Exposures: Participant-reported COVID-19 vaccination.
Main outcomes and measures: Participant-reported adverse effects and adverse effect severity. Candidate factors in multivariable logistic regression models included age, sex, race, ethnicity, subjective social status, prior COVID-19 infection, medical conditions, substance use, vaccine dose, and vaccine brand.
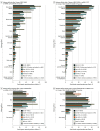
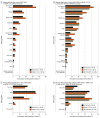
Results: The 19 586 participants had a median (IQR) age of 54 (38-66) years, and 13 420 (68.8%) were women. Allergic reaction or anaphylaxis was reported in 26 of 8680 participants (0.3%) after 1 dose of the BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna) vaccine, 27 of 11 141 (0.2%) after 2 doses of the BNT162b2 or mRNA-1273 vaccine or 1 dose of the JNJ-78436735 (Johnson & Johnson) vaccine. The strongest factors associated with adverse effects were vaccine dose (2 doses of BNT162b2 or mRNA-1273 or 1 dose of JNJ-78436735 vs 1 dose of BNT162b2 or mRNA-1273; odds ratio [OR], 3.10; 95% CI, 2.89-3.34; P < .001), vaccine brand (mRNA-1273 vs BNT162b2, OR, 2.00; 95% CI, 1.86-2.15; P < .001; JNJ-78436735 vs BNT162b2: OR, 0.64; 95% CI, 0.52-0.79; P < .001), age (per 10 years: OR, 0.74; 95% CI, 0.72-0.76; P < .001), female sex (OR, 1.65; 95% CI, 1.53-1.78; P < .001), and having had COVID-19 before vaccination (OR, 2.17; 95% CI, 1.77-2.66; P < .001).
Conclusions and relevance: In this real-world cohort, serious COVID-19 vaccine adverse effects were rare and comparisons across brands could be made, revealing that full vaccination dose, vaccine brand, younger age, female sex, and having had COVID-19 before vaccination were associated with greater odds of adverse effects. Large digital cohort studies may provide a mechanism for independent postmarket surveillance of drugs and devices.
Conflict of interest statement
Figures
References
-
- US Food and Drug Administration . Pfizer-BioNTech COVID-19 vaccine emergency use authorization. Accessed May 21, 2021. https://www.fda.gov/media/144412/download
-
- US Food and Drug Administration . Moderna COVID-19 vaccine emergency use authorization. Accessed May 21, 2021. https://www.fda.gov/media/144636/download
-
- Holder J. Tracking coronavirus vaccinations around the world. The New York Times. Accessed June 3, 2021. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracke...
-
- Kirzinger A, Kearney A, Hamel L, Brodie M. KFF/The Washington Post Frontline Health Care Workers Survey. Published March 19, 2021. Accessed June 3, 2021. https://www.kff.org/report-section/kff-washington-post-frontline-health-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

