An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis
- PMID: 34936041
- PMCID: PMC11072026
- DOI: 10.1007/s00018-021-04079-z
An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis
Abstract
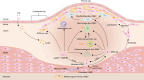
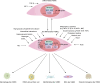
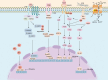
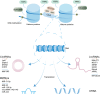
Vascular smooth muscle cells (VSMCs) are involved in phenotypic switching in atherosclerosis. This switching is characterized by VSMC dedifferentiation, migration, and transdifferentiation into other cell types. VSMC phenotypic transitions have historically been considered bidirectional processes. Cells can adopt a physiological contraction phenotype or an alternative "synthetic" phenotype in response to injury. However, recent studies, including lineage tracing and single-cell sequencing studies, have shown that VSMCs downregulate contraction markers during atherosclerosis while adopting other phenotypes, including macrophage-like, foam cell, mesenchymal stem-like, myofibroblast-like, and osteochondral-like phenotypes. However, the molecular mechanism and processes regulating the switching of VSMCs at the onset of atherosclerosis are still unclear. This systematic review aims to review the critical outstanding challenges and issues that need further investigation and summarize the current knowledge in this field.
Keywords: Atherosclerosis; Phenotypic switching; VSMCs.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Vascular smooth muscle cells in atherosclerosis: time for a re-assessment.Cardiovasc Res. 2021 Sep 28;117(11):2326-2339. doi: 10.1093/cvr/cvab046. Cardiovasc Res. 2021. PMID: 33576407 Free PMC article. Review.
-
Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Krüppel-Like Factor 4).Arterioscler Thromb Vasc Biol. 2021 Nov;41(11):2693-2707. doi: 10.1161/ATVBAHA.121.316600. Epub 2021 Sep 2. Arterioscler Thromb Vasc Biol. 2021. PMID: 34470477 Free PMC article. Review.
-
NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis.Int J Mol Sci. 2021 Dec 29;23(1):340. doi: 10.3390/ijms23010340. Int J Mol Sci. 2021. PMID: 35008765 Free PMC article.
-
Vascular Smooth Muscle Cells in Atherosclerosis.Circ Res. 2016 Feb 19;118(4):692-702. doi: 10.1161/CIRCRESAHA.115.306361. Circ Res. 2016. PMID: 26892967 Free PMC article. Review.
-
Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis.J Am Heart Assoc. 2023 Oct 17;12(20):e031121. doi: 10.1161/JAHA.123.031121. Epub 2023 Oct 10. J Am Heart Assoc. 2023. PMID: 37815057 Free PMC article. Review.
Cited by
-
The Cdc42/Rac1 pathway: a molecular mechanism behind iron-deficiency-driven aortic medial degeneration.Am J Transl Res. 2024 Aug 15;16(8):3922-3937. doi: 10.62347/YISX1726. eCollection 2024. Am J Transl Res. 2024. PMID: 39262709 Free PMC article.
-
Model Systems to Study the Mechanism of Vascular Aging.Int J Mol Sci. 2023 Oct 19;24(20):15379. doi: 10.3390/ijms242015379. Int J Mol Sci. 2023. PMID: 37895059 Free PMC article. Review.
-
Dehydrocorydaline maintains the vascular smooth muscle cell contractile phenotype by upregulating Spta1.Acta Pharmacol Sin. 2025 May;46(5):1303-1316. doi: 10.1038/s41401-024-01464-9. Epub 2025 Jan 20. Acta Pharmacol Sin. 2025. PMID: 39833304 Free PMC article.
-
Single-cell RNA sequencing to identify cellular heterogeneity and targets in cardiovascular diseases: from bench to bedside.Basic Res Cardiol. 2023 Feb 7;118(1):7. doi: 10.1007/s00395-022-00972-1. Basic Res Cardiol. 2023. PMID: 36750503 Review.
-
Fibroblast Growth Factor 21 Promotes Vascular Smooth Muscle Cell Contractile Polarization via p38 Mitogen-Activated Protein Kinase-Promoted Serum Response Factor Phosphorylation.Research (Wash D C). 2025 Aug 5;8:0815. doi: 10.34133/research.0815. eCollection 2025. Research (Wash D C). 2025. PMID: 40765997 Free PMC article.
References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

