Secular and Regional Trends among Pulmonary Arterial Hypertension Clinical Trial Participants
- PMID: 34936541
- PMCID: PMC9169130
- DOI: 10.1513/AnnalsATS.202110-1139OC
Secular and Regional Trends among Pulmonary Arterial Hypertension Clinical Trial Participants
Abstract
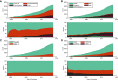
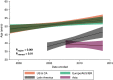
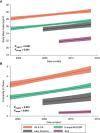
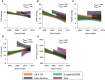
Rationale: The population of patients with pulmonary arterial hypertension (PAH) has evolved over time from predominantly young White women to an older, more racially diverse and obese population. Whether these changes are reflected in clinical trials is not known. Objectives: To determine secular and regional trends among PAH trial participants. Methods: We performed a pooled cohort analysis using harmonized data from phase III clinical trials of PAH therapies submitted to the U.S. Food and Drug Administration. We used mixed-effects linear and logistic regression to assess regional differences in participant age, sex, body habitus, and hemodynamics over time. Results: A total of 6,599 participants were enrolled in 18 trials between 1998 and 2013; 78% were female. The mean age of participants in North America, Europe, and Latin America at the time of study start increased by 2.09 (95% confidence interval [CI], 0.67-3.51), 1.62 (95% CI, 0.24-3.00), and 4.75 (95% CI, 2.29-7.21) years per 5 years, respectively (P = 0.01). Body mass index at the time of study start increased by 0.72 kg/m2 per 5 years (95% CI, 0.44-0.99; P < 0.001) across all regions. Eighty-five percent of participants in early studies were non-Hispanic White, but this decreased over time to 70%. Ninety-seven percent of Asians and 74% of Hispanics in the sample were recruited from Asia and Latin America. Conclusions: Patients enrolled in more recent PAH therapy trials are older and more obese, mirroring the changing epidemiology of observational cohorts. However, these trends varied by geographic region. PAH cohorts remain predominantly female, presenting challenges for generalizability to male patients. Although the proportion of non-White participants increased over time, this was primarily through recruitment in Asia and Latin America.
Keywords: clinical trial participants; pooled cohort analysis; pulmonary arterial hypertension; secular trends.
Figures

References
-
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. Primary Pulmonary Hypertension Study Group A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med . 1996;334:296–301. - PubMed
-
- Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med . 1987;107:216–223. - PubMed
-
- Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JSR, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med . 2012;186:790–796. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

