Implementation of Bronchoscopic Lung Volume Reduction Using One-Way Endobronchial Valves: A Retrospective Single-Centre Cohort Study
- PMID: 34937034
- PMCID: PMC9153356
- DOI: 10.1159/000520885
Implementation of Bronchoscopic Lung Volume Reduction Using One-Way Endobronchial Valves: A Retrospective Single-Centre Cohort Study
Abstract
Background: Bronchoscopic lung volume reduction (BLVR) using 1-way endobronchial valves (EBV) has become a guideline treatment in patients with advanced emphysema. Evidence from this minimally invasive treatment derives mainly from well-designed controlled trials conducted in high-volume specialized intervention centres. Little is known about real-life outcome data in hospitals setting up this novel treatment and which favourable conditions are required for a continuous successful program.
Objectives: In this study, we aim to evaluate the eligibility rate for BLVR and whether the implementation of BLVR in our academic hospital is feasible and yields clinically significant outcomes.
Method: A retrospective evaluation of patients treated with EBV between January 2016 and August 2019 was conducted. COPD assessment test (CAT), forced expiratory volume in 1 s (FEV1), residual volume (RV), and 6-min walking test (6MWT) were measured at baseline and 3 months after intervention. Paired sample t tests were performed to compare means before and after intervention.
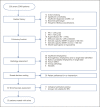
Results: Of 350 subjects screened, 283 (81%) were not suitable for intervention mostly due to lack of a target lobe. The remaining 67 subjects (19%) underwent bronchoscopic assessment, and if suitable, valves were placed in the same session. In total, 55 subjects (16%) were treated with EBV of which 10 did not have complete follow-up: 6 subjects had their valves removed because of severe pneumothorax (n = 2) or lack of benefit (n = 4) and the remaining 4 had missing follow-up data. Finally, 45 patients had complete follow-up at 3 months and showed an average change ± SD in CAT -4 ± 6 points, FEV1 +190 ± 140 mL, RV -770 ± 790 mL, and +37 ± 65 m on the 6MWT (all p < 0.001). After 1-year follow-up, 34 (76%) subjects had their EBV in situ.
Conclusion: Implementing BLVR with EBV is feasible and effective. Only 16% of screened patients were eligible, indicating that this intervention is only applicable in a small subset of highly selected subjects with advanced emphysema, and therefore a high volume of COPD patients is essential for a sustainable BLVR program.
Keywords: Bronchoscopic lung volume reduction; Emphysema; Intervention; Interventional bronchoscopy.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
R.P. has received honoraria for lectures from Health investment, Medtalks, and Chronisch Zorgnet, A.W.V. has no conflicts of interest, K.H.M.W. has no conflicts of interest, P.S.N. has no conflicts of interest, J.U.S. has no conflicts of interest, H.A.G. has no conflicts of interest, G.W. has no conflicts of interest, E.F.M.W. has no conflicts of interest, L.E.G.W.V. reports consulting fees from GSK and AstraZeneca, honoraria for lectures from GSK, AstraZeneca, Resmed, and Boehringer, and the Data Safety Monitoring Board, AstraZeneca.
Figures

References
-
- The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2020. Available from: https://goldcopd.org/gold-reports/
-
- Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med. 2000 Jul 27;343((4)):239–45. - PubMed
-
- Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE) Am J Respir Crit Care Med. 2018 Nov 1;198((9)):1151–64. - PubMed
-
- Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015 Sep 12;386((9998)):1066–73. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

