Quantifying Benefit-Risk Preferences for Heart Failure Devices: A Stated-Preference Study
- PMID: 34937393
- PMCID: PMC8763248
- DOI: 10.1161/CIRCHEARTFAILURE.121.008797
Quantifying Benefit-Risk Preferences for Heart Failure Devices: A Stated-Preference Study
Abstract
Background: Regulatory and clinical decisions involving health technologies require judgements about relative importance of their expected benefits and risks. We sought to quantify heart-failure patients' acceptance of therapeutic risks in exchange for improved effectiveness with implantable devices.
Methods: Individuals with heart failure recruited from a national web panel or academic medical center completed a web-based discrete-choice experiment survey in which they were randomized to one of 40 blocks of 8 experimentally controlled choice questions comprised of 2 device scenarios and a no-device scenario. Device scenarios offered an additional year of physical functioning equivalent to New York Heart Association class III or a year with improved (ie, class II) symptoms, or both, with 30-day mortality risks ranging from 0% to 15%, in-hospital complication risks ranging from 0% to 40%, and a remote adjustment device feature. Logit-based regression models fit participants' choices as a function of health outcomes, risks and remote adjustment.
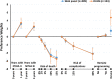
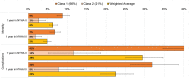
Results: Latent-class analysis of 613 participants (mean age, 65; 49% female) revealed that two-thirds were best represented by a pro-device, more risk-tolerant class, accepting up to 9% (95% CI, 7%-11%) absolute risk of device-associated mortality for a one-year gain in improved functioning (New York Heart Association class II). Approximately 20% were best represented by a less risk-tolerant class, accepting a maximum device-associated mortality risk of 3% (95% CI, 1%-4%) for the same benefit. The remaining class had strong antidevice preferences, thus maximum-acceptable risk was not calculated.
Conclusions: Quantitative evidence on benefit-risk tradeoffs for implantable heart-failure device profiles may facilitate incorporating patients' views during product development, regulatory decision-making, and clinical practice.
Keywords: decision making; heart failure; patient preference; risk assessment; surveys and questionnaires.
Figures


References
-
- Griffin JM, Borlaug BA, Komtebedde J, Litwin SE, Shah SJ, Kaye DM, Hoendermis E, Hasenfuß G, Gustafsson F, Wolsk E, et al. . Impact of interatrial shunts on invasive hemodynamics and exercise tolerance in patients with heart failure. J Am Heart Assoc. 2020; 9:e016760. doi: 10.1161/JAHA.120.016760 - PMC - PubMed
-
- Kaye DM, Hasenfuß G, Neuzil P, Post MC, Doughty R, Trochu JN, Kolodziej A, Westenfeld R, Penicka M, Rosenberg M, et al. . One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016; 9:e003662. doi: 10.1161/CIRCHEARTFAILURE.116.003662 - PMC - PubMed
-
- Karki R, Friedman PA, Killu AM. The future of percutaneous epicardial interventions. Card Electrophysiol Clin. 2020; 12:419–430. doi: 10.1016/j.ccep.2020.04.007 - PubMed
-
- Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, Abraham WT. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2020; 76:1–13. doi: 10.1016/j.jacc.2020.05.015 - PubMed
-
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health and Center for Biologics Evaluation and Research. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling: Guidance for Industry, Food and Drug Administration Staff, and other Stakeholders. Available at: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/.... Issued August 24, 2016
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

